1. Background
Schizophrenia is one of the most serious mental disorders presenting in adolescence. It has persistent and recurring nature. The lifetime prevalence is between 0.05 and 0.6%. The patients have a disability and social problems that could cause family and social disruption (1). Schizophrenia can impair cognitive function and cause other abnormal behaviors (2, 3). According to symptoms, schizophrenia is divided into two types. Schizophrenia type I presents symptoms such as hallucinations and delusions (positive symptoms), and schizophrenia type II presents with social functioning deficits, flat affect, lack of motivation, and alogia (negative symptoms). These two syndromes may coincide in a single patient. In other words, a patient can have both positive and negative symptoms at the same time. However, the therapeutic response to neuroleptic drugs is better in patients with positive symptoms than in those with negative symptoms (4). Schizophrenia is associated with mortality and economic burden (5, 6). There are still ambiguous points in the etiology of schizophrenia. In patients with schizophrenia, the non-enzymatic antioxidants decrease, and lipid peroxides and nitric oxides increase (7). Oxidative stress, membrane defects, immune system dysfunction, and pathologies of various neurotransmitter systems have been implicated in the etiology of schizophrenia. Also, the pathophysiology of negative symptoms associated with cognitive deficits is still unclear (8).
Antipsychotic drugs are the basis of treatment and clinical management of patients with schizophrenia. Despite the efficacy of risperidone as one of the antipsychotic drugs in patients with schizophrenia, there are still some problems, and the need for new therapeutic compounds for these patients is warranted. A significant number of patients remain symptomatic. About two-thirds of the patients may experience both positive and negative symptoms during their life. This indicates the inadequacy of existing treatments and the need for designing new therapies (9, 10).
Although many antipsychotic drugs are currently available, responses to these drugs vary, and finding safe, more effective, and less adversative effect drugs remains a challenge in treating these patients (1, 11). The use of alternative therapies has been suggested for patients with schizophrenia. Atypical antipsychotic drugs that are newer have fewer motor side effects than typical antipsychotic drugs. However, despite this superiority, patients have only had a slight advantage in treating negative symptoms. There are no effective treatments for treating the negative symptoms as the most damaging symptoms of schizophrenia. Researchers are trying to find drugs that have a greater impact on the treatment of negative symptoms of schizophrenia (12). Recently, schizophrenia has been linked to changes in the muscarinic system of acetylcholine. The muscarinic hypothesis of schizophrenia claims that acetylcholine plays an important role in the pathology and treatment of schizophrenia. Data from clinical studies, post-mortem studies, neuroimaging, and pre-clinical and clinical pharmacology studies support this hypothesis. Post-mortem and neuroimaging studies have shown a decrease in the number of M1 and M4 acetylcholine receptors in people with schizophrenia in various areas, including the caudate nucleus, putamen, hippocampus, anterior and posterior cingulate cortex, and prefrontal cortex. Different pharmacological approaches (e.g., increased intra-synaptic acetylcholine concentration, agonist and antagonist effects on muscarinic receptors) can be used to target the muscarinic system (13).
Donepezil, rivastigmine, and galantamine are anticholinesterase inhibitors used to treat mild to moderate cognitive deficits in Alzheimer's dementia. These drugs reduce the inactivation of the neurotransmitter acetylcholine, resulting in a moderate improvement in memory and targeted thinking. Galantamine selectively inhibits the acetylcholinesterase enzyme, as well as producing allosteric fusion of nicotine receptors. Nicotinic receptors are located in the presynaptic and postsynaptic regions of neurons. Presynaptic nicotinic receptors regulate the release of acetylcholine, glutamate, and GABA. Postsynaptic nicotine receptors mediate cholinergic transmission to the hippocampus and cortex. Both types of receptors play essential roles in memory and learning. Galantamine is a codeine-like alkaloid derived from the plant Galanthus nivalis (14). It is easily absorbed and reaches its maximum plasma concentration after five minutes to four hours. Side effects of galantamine are mild and transient and include dizziness, headache, nausea, vomiting, diarrhea, and anorexia (13).
2. Objectives
In the current study, we aimed to evaluate the efficacy of galantamine as an adjunctive treatment in ameliorating the negative symptoms of schizophrenia patients.
3. Methods
3.1. Study Design
This is a randomized, double-blind phase II clinical trial study carried out on schizophrenia patients admitted to the Psychiatric Ward of Golestan hospital, Ahwaz Jundishapur University of Medical Sciences. Schizophrenia was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders (5th Edition) (DSM-5) by an expert psychiatrist. The selection of patients was done with a random sampling strategy and according to inclusion and exclusion criteria, as shown in Table 1. A block randomization method was used for the ease of implementation and balancing the number of studied groups (intervention and placebo). The trial was approved by the Ethics Committee of Ahwaz Jundishapur University of Medical Sciences. Informed consent was obtained from all participants.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Diagnosis of schizophrenia (according to DSM-5 criteria) | Patients with other disorders of axis 1 (Hamilton-scale depression rejection) |
| Age 18 - 55 years | Patients with other uncontrolled medical disorders (neurological, cardiac, hepatic, renal, metabolic, and endocrine) |
| Receiving at least one second-generation antipsychotic drug | History of sensitivity to second-generation antipsychotic drugs or anticholinesterase drugs |
| Having a score of at least 15 in the subgroup of negative symptoms based on the PANSS | Severe drug side effects |
| Positive amphetamine test with methamphetamine on admission | |
| Recent history of amphetamine use | |
| Pregnancy or lactation | |
| Simultaneous treatment with other anticholinesterase drugs | |
| Active suicidal thought |
Inclusion and Exclusion Criteria for Patient Selection
3.2. Interventions
The patients were randomly divided into two equal groups using a block randomization method. The researcher had no role in the treatment plan or the completion of the questionnaires. Both responsible researchers and patients were not aware of the type of treatment. Patients were tracked using codes. The placebo was similar to galantamine in shape, size, color, and odor. First, a clinical demographic questionnaire was completed based on patient information and patient records, including age, gender, marital status, educational level, underlying disease, and smoking. The intervention group received 24 mg galantamine plus 2 - 6 mg risperidone, and controls received 24 mg placebo along with 2 - 6 mg risperidone. Galantamine initially started at a dose of 4 mg twice daily, gradually increasing to 12 mg twice daily for a week and continuing until the end of the week. At baseline, second, fourth, and eighth weeks after the intervention, the patients were evaluated for negative symptoms based on the SANS and PANSS, as well as the overall score of the PANSS and adverse events. Patients that experienced severe side effects were excluded.
3.3. Outcome
The effect of galantamine on negative, positive, and general symptoms of schizophrenia was a primary outcome. To evaluate the initial outcome, we used the following neurological tests at baseline and weeks 2, 4, and 8 after treatment. The Scale for the Assessment of Negative Symptoms (SANS) and the Positive and Negative Syndrome Scale (PANSS) were used for scoring the patients' symptoms (15). The secondary outcome of this study was the side effects of galantamine compared to the placebo.
3.4. Statistical Analysis
According to Conley et al. (16), by considering an error level of 0.05 and power of 90%, the final sample size was calculated to be 28 patients. All data were analyzed by descriptive statistics, including mean, median, standard deviation, and frequency. The normality of data was assessed using the Kolmogorov-Smirnov test. Based on normality, the means were compared using a t-test or Mann-Whitney test. The chi-square test was used to compare the proportions. Pearson correlation coefficient and regression analysis were used to investigate the quantitative relationships. A p value of less than 0.05 was considered significant. All data analyses were performed using SPSS version 22.
4. Results
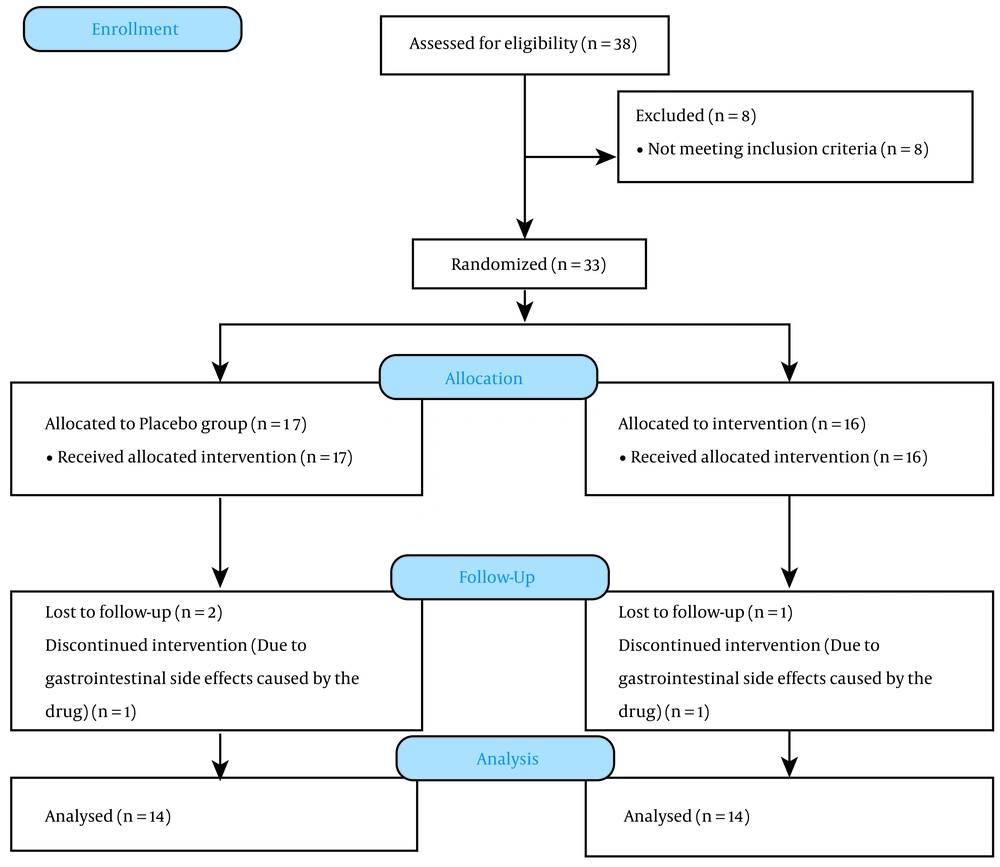
We included 38 subjects in the evaluation based on the inclusion and exclusion criteria. Eight patients were excluded from the study, and 33 patients remained. Three patients were excluded from the intervention due to inadequate follow-up conditions and the impossibility of cooperation, and the study continued with 30 patients. One male from the placebo group and one female from the galantamine group were excluded due to gastrointestinal side effects caused by the drug. Finally, 28 patients completed the follow-up (Figure 1). The mean age of the participants was 44 ± 3.1 and 47 ± 2.7 years in the placebo and intervention groups, respectively. The male to female ratios were 1: 1 and 3: 4 in the placebo and intervention groups, respectively. The patients did not show any significant differences in terms of demographic factors (P > 0.05).
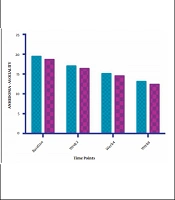
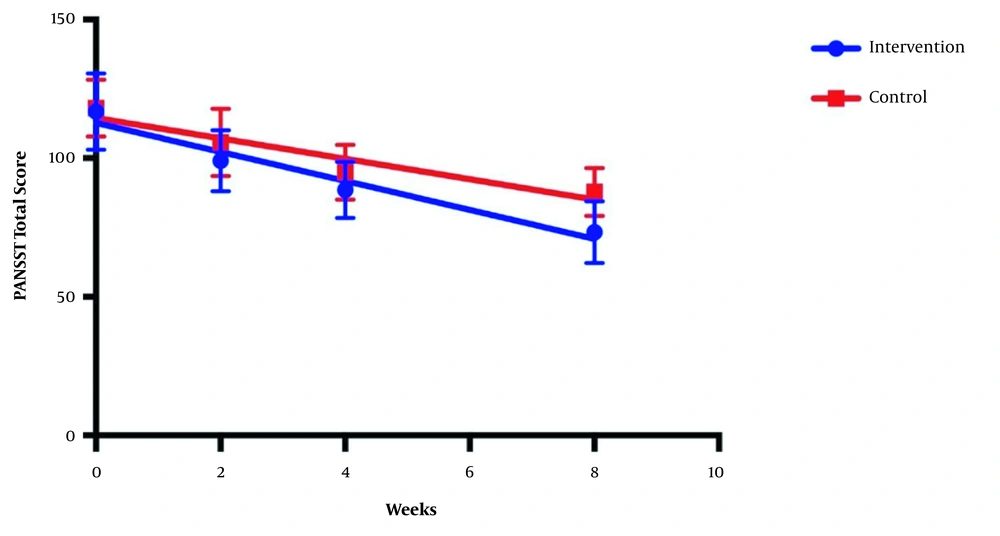
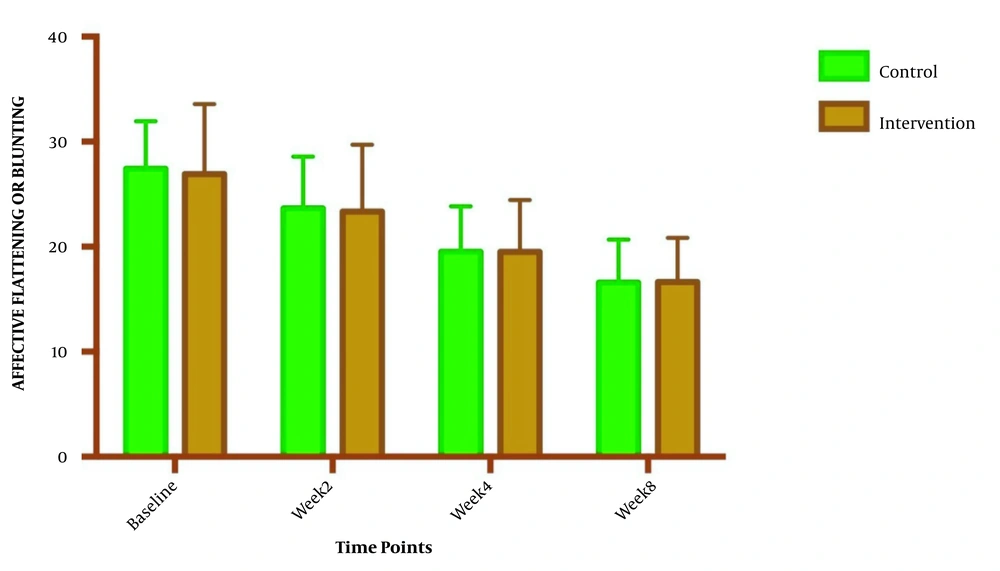
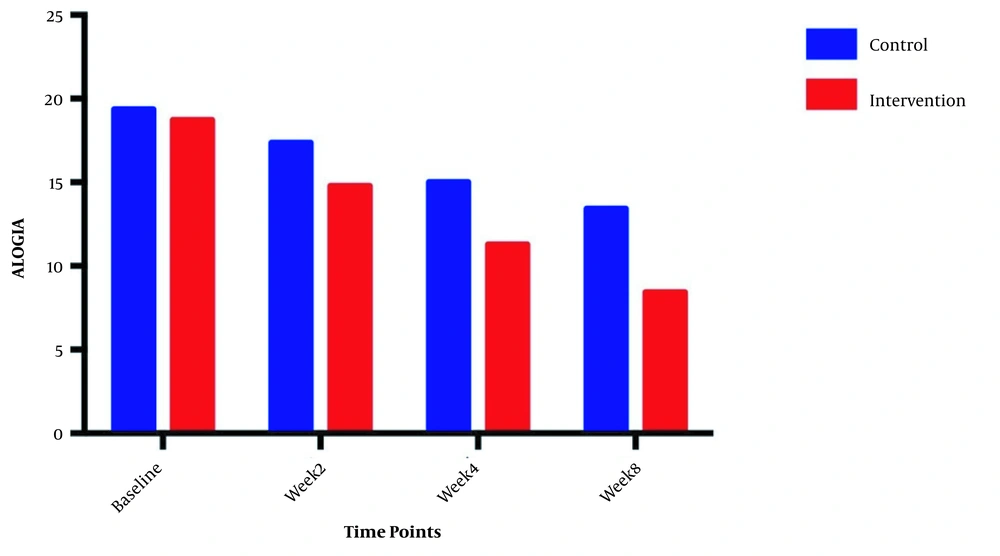
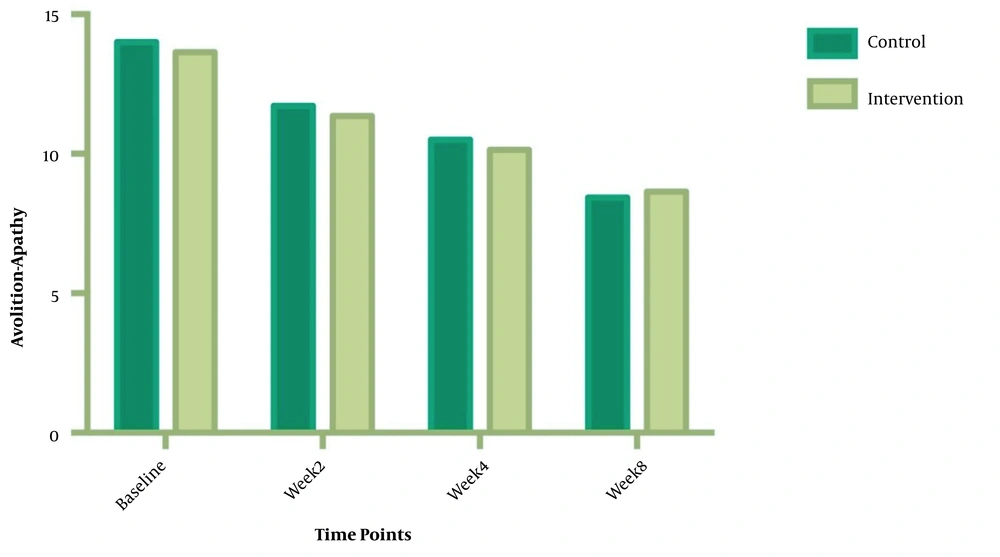
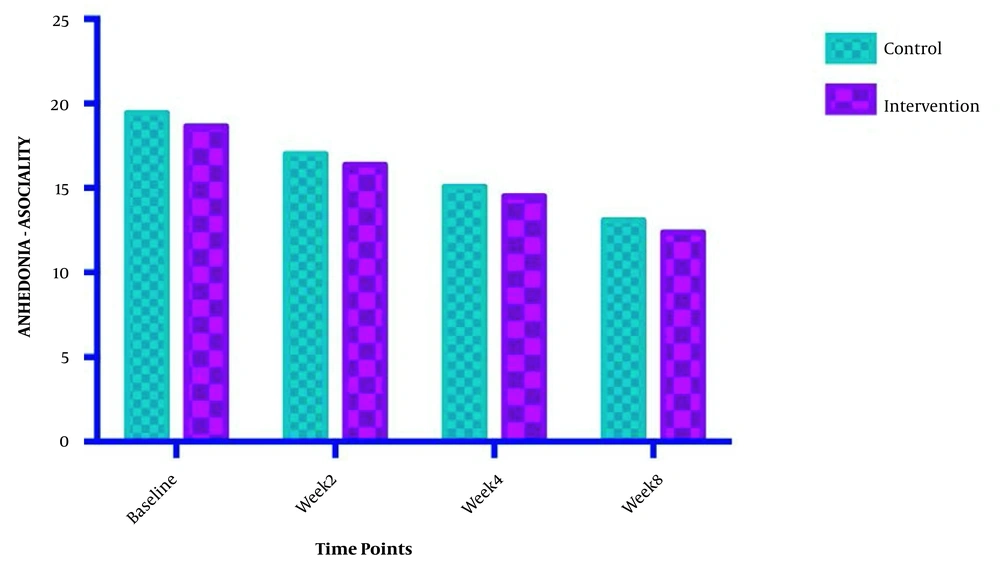
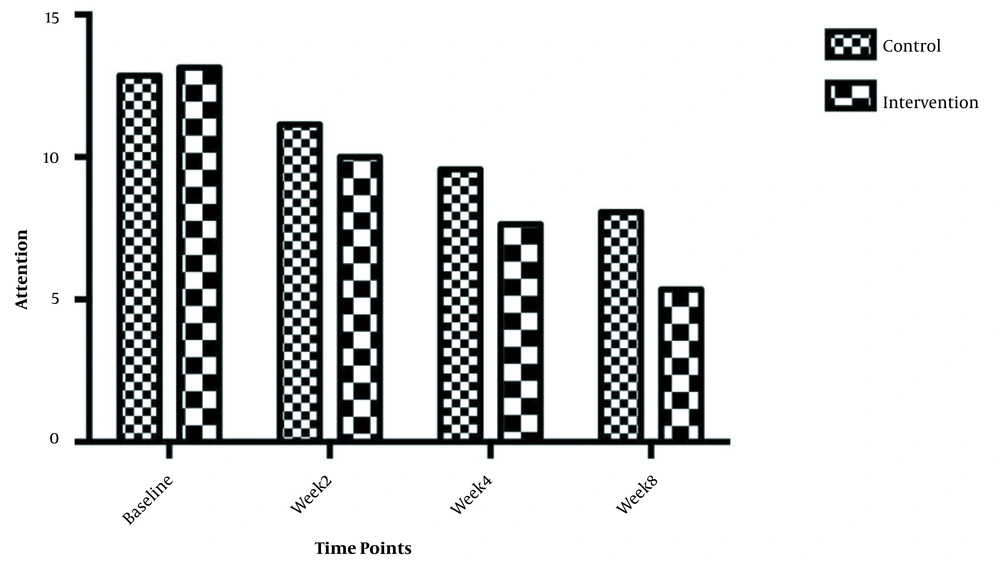
The symptom severity of patients, as the PANSS score, was evaluated at four various times. The PANSS score significantly decreased over time. Reduction slopes were compared using a linear regression model. The intervention group showed significantly greater reductions than the control group (P = 0.034) (Table 2 and Figure 2). The changes in the severity of negative symptoms are shown in Table 3. All negative symptoms significantly decreased in both groups. Although reduction slopes in affective flattening, avolition-apathy, and anhedonia-sociality symptoms did not show any significant differences between the groups, alogia and attention reduced more intensely in the intervention group than in the control group (Figures 2 - 7). The differences were statistically significant (Table 3).
| Variables | Intervention | Control | Slope Comparison |
|---|---|---|---|
| PANSS Repeated measure, mean ± SEM | |||
| Baseline | 116.8 ± 3.7 | 118.1 ± 2.7 | |
| Week 2 | 99.1 ± 3.0 | 105.6 ± 3.3 | |
| Week 4 | 88.5 ± 2.7 | 94.9 ± 2.6 | |
| Week 8 | 73.4 ± 3.0 | 87.9 ± 2.3 | |
| Linear regression | F = 4.56711; DFn = 1; DFd = 108; P = 0.0348 | ||
| Slope | -5.58 ± 0.62 | -3.9 ± 0.55 | |
| Y-intercept when X = 0.0 | 115.4 ± 2.8 | 116.3 ± 2.5 | |
| X-intercept when Y = 0.0 | 20.65 | 29.73 | |
| 1/slope | -0.17 | -0.25 | |
| Is slope significantly non-zero? | |||
| F | 80.01 | 49.76 | |
| DFn, DFd | 1, 54 | 1, 54 | |
| P value | < 0.0001 | < 0.0001 | |
| Deviation from zero | Significant | Significant |
Positive and Negative Syndrome Scale (PANSS) Score Changes During Study Time
| Symptoms/Time Points | Intervention | Control | Are the Slope Equal? |
|---|---|---|---|
| Affective Flattening | F = 0.0479981; DFn = 1; DFd = 108; P = 0.827 | ||
| Baseline | 26.93 ± 6.662 | 27.43 ± 4.536 | |
| Week 2 | 23.36 ± 6.344 | 23.64 ± 4.955 | |
| Week 4 | 19.50 ± 4.958 | 19.50 ± 4.363 | |
| Week 8 | 16.64 ± 4.217 | 16.57 ± 4.108 | |
| Is slope significantly non- zero? | < 0.0001 | < 0.0001 | |
| Alogia | F = 10.5231; DFn = 1 DFd = 108; P = 0.0016 | ||
| Baseline | 18.71 ± 1.899 | 19.36 ± 1.499 | |
| Week 2 | 14.79 ± 2.778 | 17.36 ± 2.098 | |
| Week 4 | 11.29 ± 2.998 | 15.00 ± 2.112 | |
| Week 8 | 8.429 ± 3.502 | 13.43 ± 1.869 | |
| Is slope significantly non- zero? | < 0.0001 | < 0.0001 | |
| Avolition-Apathy | F = 0.217039; DFn = 1; DFd = 108; P = 0.6422 | ||
| Baseline | 13.64 ± 2.845 | 14.14 ± 2.349 | |
| Week 2 | 11.36 ± 2.530 | 11.93 ± 2.674 | |
| Week 4 | 10.14 ± 2.825 | 10.50 ± 2.594 | |
| Week 8 | 8.643 ± 2.405 | 8.571 ± 1.869 | |
| Is slope significantly non- zero? | < 0.0001 | < 0.0001 | |
| Anhedonia-asociality | F = 0.00133612; DFn = 1; DFd = 108 | ||
| Baseline | 18.64 ± 3.522 | 19.43 ± 2.980 | |
| Week 2 | 16.36 ± 2.373 | 17.00 ± 2.572 | |
| Week 4 | 14.50 ± 2.504 | 15.07 ± 2.526 | |
| Week 8 | 12.36 ± 2.274 | 13.07 ± 1.900 | |
| Is slope significantly non- zero? | < 0.0001 | < 0.0001 | |
| Attention | F = 6.73662; DFn = 1 DFd = 108; P = 0.0 | ||
| Baseline | 13.14 ± 1.460 | 12.86 ± 1.292 | |
| Week 2 | 10.00 ± 2.602 | 11.14 ± 1.657 | |
| Week 4 | 7.643 ± 2.499 | 9.571 ± 1.697 | |
| Week 8 | 5.357 ± 2.872 | 8.071 ± 2.200 | |
| Is slope significantly non- zero? | < 0.0001 | < 0.0001 |
Changes in Negative Symptoms Severity During Study a
5. Discussion
Since antipsychotic drugs have been recognized as the basis of treatment for schizophrenia, many efforts have been made to improve negative symptoms and cognitive deficits, but these drugs, even along with other therapeutic drugs, could not properly correct cognitive impairment and negative symptoms. Much of the current literature pays attention to the effect of the galantamine-memantine combination on schizophrenia for the improvement of cognitive impairment (17-19). There are contradictory findings in previous investigations concerning the effect of cholinergic drugs on the improvement of negative and psychopathological symptoms of schizophrenia. However, studies showed that continuous treatment with these drugs has the potential to reduce the risk of recurrence in these patients. In this regard, the main purpose of the current study was to evaluate the efficacy and safety of galantamine as adjuvant therapy along with antipsychotic therapy. The current study followed up two groups of patients for eight weeks to find the efficacy of galantamine as an adjunctive treatment for ameliorating the negative symptoms of schizophrenia. The reduction levels in various symptoms were compared between the groups.
Our findings indicated that the total PANSS score reduced significantly during the study, but the reduction slope was greater in those treated by galantamine. To the best of our knowledge, a few studies have reported mixed results. Lindenmayer and Khan (2011) evaluated 32 schizophrenia patients under long-acting injectable risperidone treatment. They used galantamine or placebo at a maximum of 24 mg daily for 52 weeks. They failed to find any ameliorative effects of galantine on schizophrenia patients (20). Moreover, Buchanan et al., in another RCT study on 58 patients, showed that galantamine has no significant superiority over placebo to decrease negative symptoms (21). However, Schubert et al., in a study of 16 schizophrenia patients stabilized on risperidone, showed the significant effect of galantamine as an adjunctive treatment (22). Unlike previous studies, we evaluated the galantamine efficacy differently. We did not compare the endpoint PANSS score, but we compared the reduction slopes of PANSS between the groups. The endpoint comparison could be affected by a variety of factors. Therefore, our results can show the positive effects of galantamine in schizophrenia patients in a different way. More longer follow-up study can prove our claim. Galantamine is administered at higher doses as a nonselective cholinesterase inhibitor, while at lower doses, it acts as a relatively selective, allosteric modulator at nicotinic α4 β2 α7 receptors. Galantamine is well-tolerated with the optimal dose of 24 mg/day. It has a low clearance rate, moderate distribution, and low plasma protein binding. Its half-life in man is approximately 8 h (23).
Moreover, our findings showed that galantamine could ameliorate negative symptoms, including attention and alogia. Galantamine with the modulation of nicotinic acetylcholine receptors improves cognitive impairment, which is one of the main pathogenesis in schizophrenia. Detailed examination by Wang et al. showed that galantine and risperidone, by synergistically promoting the nicotinic acetylcholine receptors activation, can increase dopamine D1 receptor-mediated neurotransmission and affect cognitive impairments in schizophrenia patients (24). These findings are in line with previous reports. In an RCT study, Conley et al. (2009) evaluated the potential effects of galantamine on negative symptoms in 86 schizophrenia patients. They found a significant improvement in alogia after 12 weeks of treatment with 24 mg/day galantamine (16). As the main aspect of the negative symptom constructs in schizophrenia, alogia is related to cognitive impairments. It is thought that alogia is developed through semantic memory disorganization. Hence, the improvement of alogia by galantamine treatment is due to the re-establishment of verbal memory (25). Schubert et al., similar to our findings, showed the significant effects of galantamine on attention (22). Galantamine affects cognitive function by the modulation of nicotinic receptors, which may positively impact attention (26).
5.1. Limitations
The short follow-up and small sample size limited the current study. Further investigations with larger sample size and long-term follow-up need to be carried out.
5.2. Conclusion
Our findings indicated that galantamine could significantly affect the severity of the symptoms of schizophrenia patients. The finding suggests galantine as an appropriate adjunctive treatment for ameliorating negative symptoms, especially attention and alogia.