1. Background
According to the International Association for the Study of Pain (IASP), chronic pain is a status that persists or recurs for more than three months (1). Chronic pain is a debilitating condition that afflicts people with a variety of psychological distress and affects different aspects of their lives. Pain is the most common physical symptom in primary care and is considered a huge burden of suffering for patients. Moreover, it leads to work and social disability, reduces the quality of life and health care, and causes huge social and economic costs (2, 3). Psychological factors are among the factors that play important roles in the transformation of acute pain into chronic pain and its continuation, as well as in explaining the severity of pain and related problems (depression, physical disability, and anxiety) (4). Despite the positive effect of exercise on physical function and mental health (5), pain is a pervasive and disabling barrier for the injured athlete threatening his/her ability to participate in sporting events and professional goals (6); it can also cause psychological problems among them (7). Evidence show that the prevalence of chronic pain is higher in athletes than in non-athletes (8). Also, the athletes’ reactions to pain may include such symptoms as depression and suicidal thoughts (9).
Patients with chronic pain usually experience depression, disturbances in interpersonal relationships (especially in family relationships), sleep disturbances, fatigue, and decreased physical and psychological functions (10). In the chronic disease remedy, adjustment is a main factor to involve patients in the treatment process (11). Mental fatigue is among the variables that can affect people’s adjustment to their illness and increase the duration and severity of the psychological consequences. Fatigue is an unpleasant mental feeling that forms a range of feelings of physical, emotional, cognitive, to burnout that interferes with the ability to play a role in a person’s general functioning, and it is not relieved by adequate rest and sleep (12). Fatigue is one of the most common and distressing consequences of long-term complications of chronic problems, especially chronic pain (13). If the symptoms of fatigue in patients are not properly managed, they might interfere with mood, social role and function, the ability to tolerate and continue treatment, and overall quality of life (14). Research showed that athletes face stressors such as physical and mental fatigue, risk of injury, etc. (15).
Pain self-efficacy refers to a person’s confidence in their ability to maintain their function despite pain (16), which is one of the important psychological variables in these patients and athletes with chronic pain. The results of studies show that pain self-efficacy moderates the effects of frustration in patients (17). Some studies also showed that pain-related self-efficacy can directly and indirectly predict the quality of life in patients with rheumatism (18) and enhance patients’ physical activity. Therefore, pain self-efficacy can predict pain and pain-related disabilities and fatigue in patients with chronic pain (19). Research results indicate that high coping self-efficacy athletes were better able to cope with the acute stressor, adjust their behaviors in a timely manner according to the results of their coping, and focus more on processing positive information (20).
Chronic pain can lead to drug addiction and cause emotional distress for patients (2). Since emotional and cognitive problems can increase the persistence of psychological damage caused by the disease, emotion regulation can be one of the important issues, which can provide the mental health of these patients. Emotion regulation plays an important role in our adaptation to stressful life events (21, 22). Emotion-based approaches are a structured treatment program used to manage chronic pain and reduce the incidence and severity of psychological complications caused by chronic diseases (23).
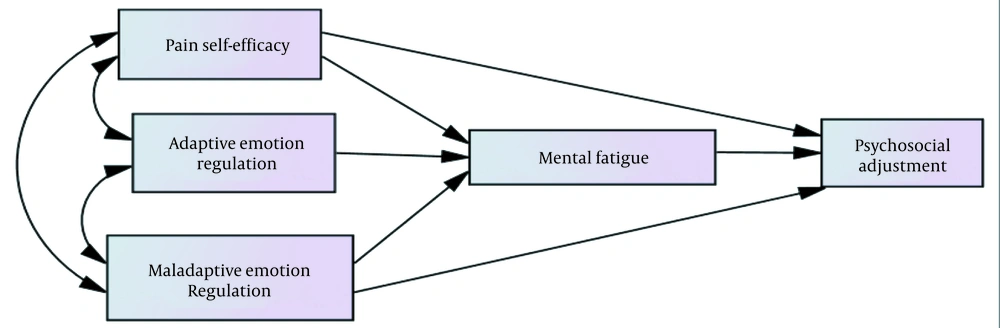
Due to the multidimensionality of the factors involved in the occurrence, persistence, and exacerbation of pain, in addition to drug therapies, improving the psychological level of patients has been recently considered to reduce the problems caused by this disease (19). Training adaptive skills to the patients and proper coping strategies are important to reduce or manage psychological stress in patients with chronic pain. Therefore, addressing the role of psychological variables to increase adjustment and reduce psychological fatigue in patients with chronic pain seems essential. Hence, the present research aimed to investigate the direct and indirect relationship between mental fatigue, pain self-efficacy, and emotion regulation with psychosocial adjustment in athletes with chronic pain. The conceptual model of the study is shown in Figure 1.
2. Objectives
The present study aimed to investigate the mediating role of mental fatigue in the relationship between emotion regulation and pain self-efficacy with psychosocial adjustment in athletes with chronic pain.
3. Methods
This descriptive-correlational study investigated athletes with chronic pain referred to the Iranian Sports Medicine Federation in 2019. In accordance with the proposed sample size for path analysis and regression analysis (24), 200 athletes with chronic pain were selected by convenience sampling method. Inclusion criteria were at least five years of professional sports background; Iranian nationality; living in Tehran city; diagnosis of chronic pain; and willingness to participate in the study. For ethical considerations, we obtained an informed consent from all participants and assured the confidentiality of their information.
3.1. Research Tools
3.1.1. Psychosocial Adjustment to Illness Scale (PAIS)
This scale was developed in 1990 by Leonardo Drugatis to evaluate psychosocial adjustment to illness during chronic illness. It contains 46 questions and seven subscales on a 4-point Likert scale (never = 0, slightly = 1, to some extent = 2, and completely = 3). Scoring is based on 0 to 3, and a higher score indicates poor adjustment. This scale was translated and standardized in Iran by Feghhi et al. in 2013, and its validity and reliability were reported as desirable (25).
3.1.2. Pain Self-efficacy Questionnaire (PSEQ)
This inventory was developed by Nicholas in 1989 and consists of 10 items which evaluate the efficiency and adequacy of people with chronic pain, and the questions are scored in a 7-point Likert scale from 0 (I am not at all sure) to 6 (I am completely sure). The patient’s score on this scale varies between zero and 60, and a higher score indicates a higher sense of self-efficacy in the population experiencing chronic pain. This questionnaire was standardized in Iran, and its validity and reliability were confirmed (19, 26).
3.1.3. Fatigue Symptom Inventory (FSI)
Fatigue score consists of 15 questions and three subgroups, including physical, emotional, and cognitive. Each question has a score between 0 (not at all) to 4 (very high), and the patient’s recent condition is marked on a questionnaire. Thus, the probable degree of fatigue in the physical dimension varies between 0 to 28; it is between 0 to 16 in the emotional dimension, between 0 to 16 in the cognitive dimension, and the overall fatigue score varies between 0 to 60 (27). In Iran, the Cronbach’s coefficient of the questionnaire was reported to be 0.92, 0.89, 0.85, and 0.95 in different dimensions of physical, emotional, cognitive, and total fatigue scores, respectively, which is satisfactory (28).
3.1.4. Cognitive Emotion Regulation Questionnaire (CERQ-short)
This questionnaire was developed by Garnefski & Kraaij (29). It is a multidimensional questionnaire and a self-report tool with 18 items (29). It has nine subscales, and the scores range from 1 (almost never) to 5 (almost always). The alpha coefficient for the subscales of this questionnaire was reported to be in the range of 0.71 to 0.81 by Garnefski & Kraaij (29). Also, the psychometric properties of this questionnaire in Iran showed that it had good validity and reliability (30).
Data were analyzed by correlation matrix, regression analysis, and path analysis method using SPSS (v21) and AMOS (v23) software at the significance level α = 0.05.
4. Results
All the 200 participants in this study (age range: 10 - 60 years) were individuals with a history of continuous exercise currently suffering from chronic pain. Among the participants, 25.83% underwent surgery, 38.33% physiotherapy, 13.33% medication, 4.16% traditional medicine, 4.16% laser treatment, and 14.16% underwent massage to improve their disease.
As can be seen in Table 1, the mean and standard deviation of psychosocial adjustment, pain self-efficacy, maladaptive emotion regulation, adaptive emotion regulation, and mental fatigue were 54.13 ± 9.71, 48.67 ± 4.40, 20.77 ± 3.31, 33.19 ± 3.35, and 36.67 ± 2.15, respectively (Table 1). The correlation matrix between the research variables showed that pain self-efficacy had a negative relationship with mental fatigue and positive relationship with psychosocial adjustment (P < 0.01). Maladaptive emotion had a positive relationship with mental fatigue but a negative relationship with psychosocial adjustment (P < 0.01). While adaptive emotion had a negative relationship with mental fatigue, it had a positive relationship with psychosocial adjustment (P < 0.01). Mental fatigue had a negative and significant relationship with psychosocial adjustment (P < 0.01).
aP < 0.05
bP < 0.01
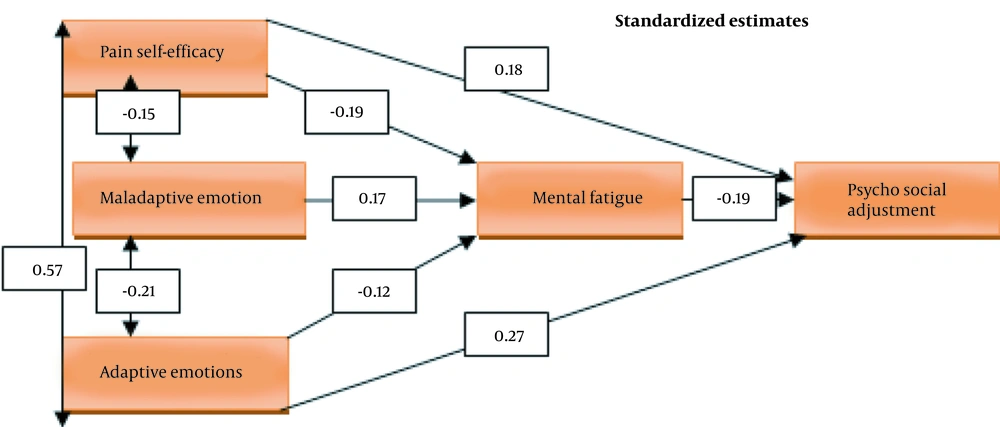
To analyze the significance of the research model, path analysis test was used. As β coefficients showed, the direct effect of pain self-efficacy, adaptive emotion regulation, and mental fatigue on psychosocial adjustment were confirmed. Also, the direct effect of pain self-efficacy, maladaptive emotion regulation, and adaptive emotion regulation on fatigue were confirmed; but the direct effect of maladaptive emotion regulation on psychosocial adjustment was not significant (Table 2). In addition, the results of the Fitness indicators of the final model indicated that the model fitted well to the data (χ2.df = 1.198, CFI = 0.998, RMSEA = 0.041).
| Variables | B | β | T | P |
|---|---|---|---|---|
| Psychosocial adjustment | ||||
| Pain self-efficacy | 0.71 | 0.18 | 2.22 | 0.016 |
| Adaptive emotion regulation | -0.27 | -0.09 | -1.09 | 0.27 |
| Maladaptive emotions | 0.77 | 0.27 | 2.71 | 0.007 |
| Mental fatigue | -0.85 | -0.19 | -2.33 | 0.025 |
| Mental fatigue | ||||
| Pain self-efficacy | -0.21 | -0.19 | -4.12 | 0.001 |
| Adaptive emotion regulation | 0.12 | 0.17 | 2.09 | 0.036 |
| Maladaptive emotions | -0.19 | -0.12 | -2.75 | 0.003 |
The indirect effect of pain self-efficacy and maladaptive emotions on psychosocial adjustment were equal to 0.036 and -0.0332, which was significant at the level of 0.05. Also, this effect was not significant in adaptive emotions; thus, the mediating role of mental fatigue in pain self-efficacy, maladaptive emotions, and psychosocial adjustment was confirmed (Table 3). Figure 2 shows the final model of research along with the paths and coefficients related to the variables in the standard mode: DF = 1, P = 0.274, χ2 = 1.198, χ2/DF = 1.198, RMSEA = 0.41, CFI = 0.998.
| Variables | Estimate Amount | Upper Line | Lower Line | Significant Level | ||
|---|---|---|---|---|---|---|
| Predictor Variables | Intermediate Variables | Criterion Variables | ||||
| Pain self-efficacy | Mental fatigue | Psychosocial adjustment to illness | 0.078 | 0.100 | 0.05 | 0.038 |
| Adaptive emotion regulation | 0.067 | 0.069 | 0.001 | 0.114 | ||
| Maladaptive emotions | -0.102 | -0.004 | -0.090 | 0.045 | ||
5. Discussion
The present study aimed to evaluate the direct and indirect relationships between mental fatigue, pain self-efficacy, and emotion regulation with psychosocial adjustment in athletes with chronic pain.
The findings showed a significant and direct effect of adaptive emotion regulation (β = 0.27) and mental fatigue (β = -0.19) on psychosocial adjustment; however, the direct effect of maladaptive emotion regulation (β = -0.09) on psychosocial adjustment was not significant. Also, the direct effects of maladaptive emotion regulation (β = 0.17) and adaptive emotion regulation (β = -0.12) on fatigue were significant. Likewise, the results of path analysis showed that the indirect effect of maladaptive emotion regulation on psychosocial adjustment was significant at the level of 0.05. Also, this effect was not significant in emotion adjustment; thus, the mediating role of mental fatigue in the regulation of maladaptive emotions and psychosocial adjustment was confirmed. Therefore, the maladaptive emotion regulation has an indirect negative effect on the psychosocial adjustment of athletes with chronic pain through increasing mental fatigue.
The results of this research are consistent with some previous studies (12-31) indicating that fatigue is associated with high levels of depression and anxiety and can affect the patient’s ability to return to normal daily life. Besharat et al. (32) also showed that the level of psychological distress had a significant negative relationship with patients’ perceived improvement. Fatigue may also occur as a decrease in motivation, lack of interest, fatigue, or as an inability to start tasks or avoid social interactions or other activities (32). Also, considering the direct and indirect effects of emotion regulation on patients’ adjustment, it can be said that emotion regulation, like other behavioral and social dimensions, is actually used to manage emotions to increase adjustment, and it is part of adjustment strategies to treat emotional and physical problems.
The results of this study also showed the significant and direct effect of pain self-efficacy on psychosocial adjustment and mental fatigue. Also, pain self-efficacy had an indirect and significant effect on psychosocial adjustment. Therefore, the mediating role of mental fatigue in pain self-efficacy and psychosocial adjustment was confirmed. Hence, pain self-efficacy, with a reverse effect on mental fatigue, can have a positive effect on the psychosocial adjustment of athletes with chronic pain. Our results were in line with the research by Knittle et al. (18), showing that pain-related self-efficacy can, directly and indirectly, predict the quality of life of patients with rheumatism (18). Boroumand et al. (26) showed that depression and suicidal thoughts had a significant correlation with chronic pain, and self-efficacy could moderate such a relationship, which was in line with our results. Feelings of self-confidence and self-efficacy in performing tasks and duties can reduce despair and consequently mental fatigue in people with chronic pain by increasing positive feelings. Pain self-efficacy can predict pain and pain-related disabilities and fatigue in patients with chronic pain (33). People with chronic pain who have a sense of self-efficacy in performing their activities and roles are easily able to deal with the challenges associated with chronic pain conditions. It also helps them overcome the limitations and thus increases their psychosocial adjustment.
One of the limitations of this study was that it was performed only on athletes with chronic pain. So, generalizing the results to other diseases should be done cautiously. In this regard, it is recommended to conduct similar studies on other social groups. Also, it is suggested that sports professionals consider psychological interventions and rehabilitation counseling in their treatment plans for athletes.
5.1. Conclusions
In line with several previous studies, the results of this study showed that mental fatigue plays a good mediating role between pain self-efficacy and emotion regulation with psychosocial adjustment of athletes with chronic pain.