1. Background
Anemia is common in patients with chronic kidney disease (CKD), the rising prevalence and severity of which is associated with the kidney dysfunction (1). According to the World Health Organization (WHO), the global prevalence of anemia is 24.8%, affecting about 1.62 billion people worldwide, with the highest and lowest prevalence rates in preschool children (47.4%) and men (12.7%), respectively. It is also estimated that 52% and 23% of pregnant women in developing and developed countries suffer from anemia, respectively (2). The prevalence of anemia in Iran is 23%; however, it may range from 10 to30%. In Iran, the prevalence of anemia is higher, especially in children and adolescents, women, and the elderly (3).
Furthermore, anemia is associated with a wide range of clinical signs and symptoms, leading to poor quality of life, development of microvascular complications, and mortality risk in patients (4). In CKD patients, anemia caused by erythropoietin deficiency is estimated to range from 26 to 75% when the glomerular filtration rate (GFR) drops from < 60 to < 15 mL/min (5).
The leading cause of anemia in these patients is low erythropoietin levels; however, other factors such as vitamin D deficiency also affects its progression and severity (6). Recent evidence suggests that vitamin D plays a critical role in different body systems regarding the distribution of vitamin D receptors across the body. In addition to the role of vitamin D in bones and mineral metabolism this vitamin adopts a protective role against several chronic diseases, including CKD-associated anemia (7).
Peritoneal dialysis (PD) and hemodialysis (HD) are dialysis options for patients with the end-stage renal kidney disease, also known as end-stage renal disease (ESRD), for whom preemptive kidney transplantation is not possible (8).
HD can significantly lower and stabilize blood pressure and consequently reduce morbidity and mortality rates in the CKD patients (9). HD is the most common dialysis method and aims to bring the kidney patient's life closer to everyday life. Annually, the prevalence of HD patients in Iran increases by about 15% (10). Recent studies have documented that the concentration of systemic inflammatory factors and vascular inflammatory factors is increased in HD patients (11). Hypotension is the most common HD complication. HD has many complications as follows: gastrointestinal complications such as anorexia and anemia, neurological complications, and reproductive system complications such as menstruation changes, all of which disrupt the quality of life in patients and arouse depression and sometimes suicide (12). Numerous and complex drug treatments in patients with advanced and chronic renal failure affect their social and psychological functioning, causing them to undergo hemodialysis (13).
Most HD patients suffer from vitamin D deficiency, and with the use of cholecalciferol and ergocalciferol supplements, the deficiency can be decreased by an average level (14). Ergocalciferol significantly reduces the use of erythropoietin in HD patients. Vitamin D compounds may improve the response to erythropoietin by suppressing chronic inflammation and controlling hyperparathyroidism by stimulating erythroid progenitors directly. In this regard, erythropoietin improves the treatment of anemia in HD patients (15, 16).
Given that vitamin D is involved in reducing the need for erythropoietin and the cost of receiving erythropoietin (17), on the other hand, since various studies have revealed different effects of vitamin D on anemia, the definitive effect of vitamin D on anemia in dialysis patients is not well-established (17-19).
2. Objectives
The present study aimed to evaluate the effect of vitamin D deficiency treatment on hemoglobin levels in HD patients.
3. Methods
3.1. Study Design
The present study was a double-blind, randomized clinical trial on HD patients undergoing weekly dialysis and receiving erythropoietin. These patients with vitamin D (< 30 ng/mL) referred to the dialysis wards of Imam Khomeini, Razi, and Sina hospitals in Ahvaz in 2019.
3.2. Study Population
The present study was a double-blind, randomized clinical trial on HD patients undergoing weekly dialysis and receiving erythropoietin. All patients with vitamin D (< 30 ng/mL) referred to the dialysis wards of Imam Khomeini, Razi and Sina hospitals in Ahvaz in 2019. Since there was no similar study, the present study was performed without determining the sample size in phase 2 of the clinical trial with 30 patients in each group. The inclusion criteria were patient satisfaction, patient with ESRD undergoing weekly HD and treated with erythropoietin, not taking vitamin D supplements during the last six months, and vitamin D level < 30 ng/mL. Exclusion criteria were not taking erythropoietin due to hemoglobin > 13, vitamin D level > 30 ng/mL, and patients with hypercalcemia. Vitamin D toxicity, also called hypervitaminosis D, is a rare but potentially serious condition that occurs when markedly elevated to 25(OH)D levels (> 100 ng/mL) in the body.
3.3. Grouping and Randomization
The participants were randomly assigned into groups A and B. The randomization method was based on the blockchain method, according to which individuals were randomly divided into two groups based on the first four randomized trials. The procedure was performed by a person not involved in the study process. For this purpose, six blocks of AABB, ABAB, ABBA, BAAB, BBAA, and BABA, sampled with the number of N / 4s, were considered. The patients, the interventionist, and the person who reviewed the results were unaware of the group of individuals, and the study was double-blinded. The patients with vitamin D deficiency were assigned into two groups. One group was treated with oral calcium-D tablets as the control group, and another group was treated with D-Pearls. The control group should not have been treated; however, the control group in this study received calcium D tablets since the ethics committee assumed that patients with vitamin D deficiency should not be left untreated.
3.4. Intervention Procedures
At the beginning of the study, blood samples were taken from all patients to check their hemoglobin and vitamin D levels. Patients with vitamin D deficiency were assigned into two groups. One group was treated with calcium-D oral tablets three times a day, and another group was treated with 50000 IU pearl vitamin D3 weekly. The two groups were treated for 12 weeks and then re-evaluated in terms of response to treatment, Hemoglobin improvement, and erythropoietin dose. To ensure the correct use of drugs in both groups, the pills were given to the patients by the dialysis ward nurse blindly. Vitamin D tablets were given to the patient every week by the nurse at the time of the visit. However, the calcium D tablets were needed to be taken three times a day; thus, the patient's companion was asked to give the tablets to the patient during the day.
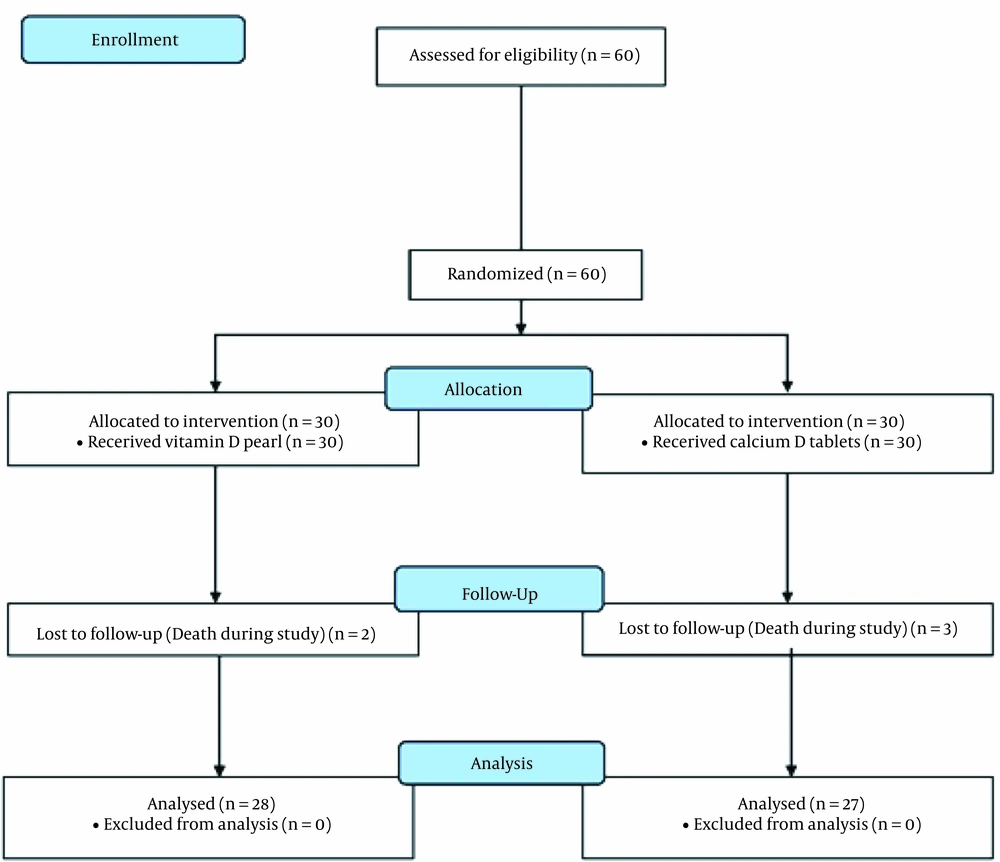
During the treatment process, the patients were visited by a nephrologist every month, and the other causes of anemia were evaluated and treated if necessary. PTH, calcium, and phosphorus were checked, and if PTH increased, the patients had treatment. The patients who became infected, passed away, and did not take the tablets completely were excluded from the study. Two and three dead patients in the vitamin D and calcium D groups were excluded from the study, respectively.
3.5. Ethical Consideration
This study was approved by the Ethics Committee of the Jundishapur University of Medical Sciences, Ahwaz (Code: IR.AJUMS.REC.1399.288). The study was listed in the Iranian Registry of Clinical Trials (Registration No. IRCT20190107042264N4).
3.6. Statistical Analysis
Mean was used to describe the quantitative variables, and standard deviation was used to describe the distribution of the data. Moreover, frequency and percentage were used to describe qualitative variables. Data normalization was assessed using Kolmogorov-Smirnov test, and the homogeneity of variances was tested by Levene's test. Since normal distribution of the data was not confirmed in this study, nonparametric tests were used to analyze the results. To compare the mean difference between the two groups (comparison between groups), the Mann-Whitney U test and the Wilcoxon test were used to compare the effectiveness of treatment in each group. Chi-square (or Fisher's) test and the Spearman’s correlation test were also used to analyze the data. Data analysis was performed using SPSS software version 22 (SPSS Inc., Chicago, IL, USA), and a P < 0.05 was set as the significance level.
4. Results
4.1. Results Before Intervention
Sixty patients participated in the study, of whom five patients (two patients from the D-Pearls group and three patients from the calcium-D group) died during the study (Figure 1). Of the remaining 55 patients, 39 were male (70.9%), and 16 (29.1%) were female. The participants’ mean age was 55.64 ± 13.26 years (18 - 83 years). Table 1 shows the characteristics of the two groups of patients. According to the study results, there was no significant difference between the study groups before the study (P < 0.05).
| Variables | Group | P-Value | |||||
|---|---|---|---|---|---|---|---|
| Vitamin D | Calcium-D | Total | |||||
| N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | ||
| Age | 28 | 54.5 ± 13.082 | 27 | 56.81 ± 13.528 | 55 | 55.64 ± 13.231 | 0.522 |
| Vitamin D | 28 | 18.21 ± 6.202 | 27 | 18 ± 8.224 | 55 | 18.11 ± 7.198 | 0.915 |
| Hb | 28 | 9.88 ± 1.715 | 27 | 9.28 ± 1.617 | 55 | 9.58 ± 1.68 | 0.187 |
| Erythropoietin dose | 27 | 104000 ± 47067.872 | 27 | 96888.89 ± 35523.918 | 54 | 100444.44 ± 41457.715 | 0.534 |
| Ca | 28 | 8.09 ± 0.898 | 27 | 8.19 ± 1.09 | 55 | 8.14 ± 0.989 | 0.703 |
| Parathyroid hormone | 28 | 679.86 ± 297.338 | 27 | 569.96 ± 247.129 | 55 | 625.91 ± 276.919 | 0.143 |
| P | 28 | 5.74 ± 1.263 | 27 | 5.74 ± 1.132 | 55 | 5.74 ± 1.189 | 0.987 |
Moreover, there was no statistically significant difference between men and women before the intervention (Table 2).
| Variables | Group | P-Value | |||||
|---|---|---|---|---|---|---|---|
| Men | Women | Total | |||||
| N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | ||
| Age | 39 | 55.72 ± 13.397 | 16 | 55.44 ± 13.246 | 55 | 55.64 ± 13.231 | 0.944 |
| Vitamin D | 39 | 18.87 ± 7.385 | 16 | 16.26 ± 6.572 | 55 | 18.11 ± 7.198 | 0.224 |
| Hb | 39 | 9.59 ± 1.707 | 16 | 9.56 ± 1.666 | 55 | 9.58 ± 1.68 | 0.939 |
| Erythropoietin dose | 38 | 97894.74 ± 35894.582 | 16 | 106500 ± 53284.144 | 54 | 100444.44 ± 41457.715 | 0.491 |
| Ca | 39 | 8.02 ± 0.911 | 16 | 8.41 ± 1.142 | 55 | 8.14 ± 0.989 | 0.187 |
| Parathyroid hormone | 39 | 604.77 ± 274.288 | 16 | 677.44 ± 285.441 | 55 | 625.91 ± 276.919 | 0.382 |
| P | 39 | 5.88 ± 1.261 | 16 | 5.39 ± 0.941 | 55 | 5.74 ± 1.189 | 0.167 |
4.2. Results After Intervention
Table 3 presents the results of comparing the measured parameters after the intervention. Regarding the studied variables, the two groups were significantly different only in terms of vitamin D levels (P < 0.001).
| Variables | Groups | P-Value | |||||
|---|---|---|---|---|---|---|---|
| Vitamin D | Calcium-D | Total | |||||
| N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | ||
| Vitamin D | 28 | 66.39 ± 19.917 | 27 | 31.97 ± 11.811 | 55 | 49.5 ± 23.813 | < 0.001 |
| Hb | 28 | 10.63 ± 1.79 | 27 | 10.52 ± 1.845 | 55 | 10.57 ± 1.801 | 0.835 |
| Erythropoietin dose | 27 | 88000 ± 50254.736 | 23 | 92521.74 ± 49246.015 | 50 | 90080 ± 49336.76 | 0.750 |
| Ca | 28 | 8.33 ± 0.836 | 27 | 8.65 ± 0.918 | 55 | 8.49 ± 0.884 | 0.177 |
| Parathyroid hormone | 28 | 639.43 ± 395.208 | 27 | 476.37 ± 260.326 | 55 | 559.38 ± 342.771 | 0.076 |
| P | 28 | 5.18 ± 1.509 | 27 | 5.22 ± 1.379 | 55 | 5.2 ± 1.434 | 0.904 |
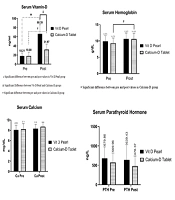
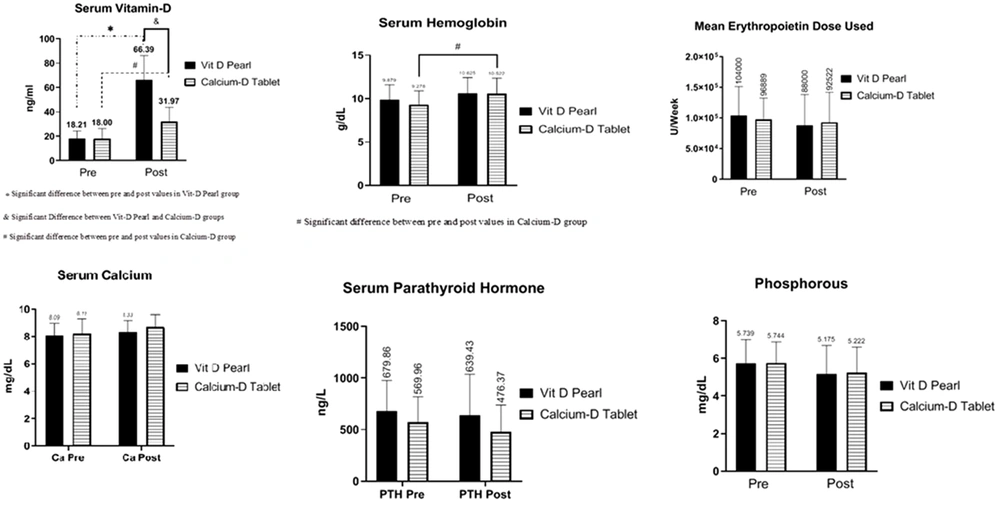
Table 3 shows that the vitamin D level in both groups increased significantly after the intervention, indicating significantly higher vitamin D level in the D-Pearls group than in the calcium-D group (P < 0.001). Before the intervention, vitamin D deficiency (vitamin D level less than 20 ng/mL) was also observed in 14 patients in the D-Pearls group (50%) and 12 patients (42.9%) in the calcium-D group (P = 0.6). After the intervention, however, no patient in the D-Pearls group and two (7.14%) patients in the calcium-D group had the deficiency. Vitamin D toxicity (above 100 ng/mL) was not observed in patients before and after the study.
The hemoglobin level in both groups increased after the intervention; however, this increase was statistically significant in the group receiving the calcium-D group (P = 0.048). Moreover, there was no statistically significant relationship between the two groups before and after the intervention regarding hemoglobin levels (P = 0.57, P = 0.99, Figure 2).
Although in each treatment group, the dose of EPO decreased after intervention compared to before intervention, the relationship was not statistically significant (P = 0.57, P = 0.98). The EPO dose before and after the intervention was not statistically significant in the vitamin D group compared to the calcium-D group (P = 0.94, P = 0.98). Moreover, the decrease in the average dosage was more significant in the vitamin D group (16,000 units vs. 4367 units). Although there was no significant difference between the two groups before and after the intervention in the dose of erythropoietin, the use of erythropoietin in the four patients (14.8%) in the calcium-D group was stopped due to the optimal hemoglobin level, while the same happened to one patient (3.57%) in the vitamin D group (P = 0.15, Figure 2).
Despite the increase in calcium levels in both groups, no significant difference was observed after the intervention (P = 0.76, P = 0.27). The calcium level before and after the intervention was not statistically significant in the vitamin D group compared to the calcium-D group (P = 0.97 and P = 0.57, respectively). However, there was no significant difference in the calcium levels between the two groups before and after the intervention. Before the intervention, hypocalcemia had an overall prevalence of 61.8% and was observed in 20 patients (71.4%) in the vitamin D group and 14 (51.8%) in the calcium-D group (P = 0.13). However, after the intervention, the prevalence was detected in 15 patients (53.6%) in the vitamin D group and eight (29.6%) in the calcium-D group (P = 0.0739). This decrease in the prevalence of hypocalcemia in each of the treatment groups was not statistically significant (P = 0.17, P = 0.1, Figure 2).
In both treatment groups, there was a decrease in parathyroid hormone levels before the intervention than after the intervention, although not statistically significant. (P = 0.96, P = 0.67). The parathyroid hormone level before and after the intervention in the vitamin D group was not statistically significant compared to the calcium-D group (P = 0.54, P = 0.20, Figure 2).
Despite the decrease in phosphorus in both groups after the intervention, no significant difference was observed (P = 0.38, P = 0.47). The difference in phosphorus levels was not statistically significant between the two groups before and after the intervention (P = 0.99, P = 0.99). The decrease in the phosphorus level was more significant in the D-Pearls group (0.56-unit decrease in the D-Pearls group vs. 0.52-unit decrease in the calcium-D group, Figure 2)
5. Discussion
This study aimed to evaluate the effect of two types of vitamins D3 therapy (namely D-Pearls and calcium-D tablets) on anemia in HD patients with ESRD. This study shows that vitamin D deficiency is widespread in dialysis patients, and that supplements can significantly improve such deficiency. This study also reveals that the weekly consumption of D-Pearls is more effective in improving vitamin D deficiency than taking calcium-D tablets three times a day. In the present study, three months after taking pearl vitamin D, all patients reached normal or above normal levels (30 ng/mL). In contrast, more than half of the patients (53.6%) did not reach the normal level in the calcium-D group despite exceeding the deficiency rate (20 ng/mL). This finding suggests that D-Pearls is a more reliable supplement when the goal of treatment is to supplement vitamin D in the short term. Lee et al. (20) provided similar results during more extended periods of ergocalciferol consumption. The study by Kim et al. (18) shows in non-dialysis patients with CKD, this rate at 3 months was 76.5% of patients and 89.7% at 6 months.
In the present study, either type of supplements did not cause a significant difference in serum calcium, phosphorus, or PTH levels. These findings were consistent with the results of studies by Blair et al. (21) and Shah et al. (22), who evaluated the level of vitamin D deficiency and the outcome of its correction in patients undergoing peritoneal dialysis.
The present findings were consistent with those reported by Miskulin et al. (23), suggesting that the mean serum level of vitamin D improved with treatment and that erythropoietin, serum calcium, phosphorus, and parathyroid hormone levels did not change. However, in the present study, calcium-D tablets were associated with an improvement in hemoglobin (above one unit). This study also showed that the effect of using D-Pearls on improving vitamin D levels was more significant than that of calcium-D tablets.
The present findings also suggested that hemoglobin levels increased in both groups; however, this increase was not significant in the D-Pearls group (P > 0.05). These findings confirm those reported by Blair et al. (21), who claimed that ergocalciferol improves hemoglobin levels. Not all patients are expected to experience a decrease in erythropoietin after taking the supplement because other factors, such as secondary hyperparathyroidism, inflammation, and infection, also affect the erythropoietin response. More than half of the patients in the present study (57%) experienced a monthly dose decrease in erythropoietin after the intervention. Although there was no significant difference between the two groups in the dose of erythropoietin before and after the intervention, the use of erythropoietin in four patients (14.8%) in the calcium-D group stopped due to reaching the optimal hemoglobin level.
In the present study, a decrease in PTH was observed after three months of vitamin D treatment in both groups; however, the decrease was much more evident in the calcium-D group, although it was not statistically significant in either group.
Although the improvement of secondary hyperparathyroidism with an increase in 25(OH)D has been reported in some studies, the findings are controversial (24-26). Several reasons for these contradictory results are as follows: differences in PTH levels in the concerned populations, differences in vitamin supplement protocol, and the improvement rate in vitamin levels (27). The mechanism of identifying PTH reduction over three months is complicated because of multiple interactions among mineral metabolism markers. In this regard, it should be noted that the PTH reduction rate was more significant in the two groups at the same time. PTH can inhibit the endogenous production of erythropoietin because erythropoietin concentrations increase after parathyroidectomy. The inhibitory effect of PTH on hematopoiesis has already been demonstrated in some studies; however, no consistent finding is reached (28).
The number of HD patients participating in this study was limited; hence, the study provided no strong evidence to detect a statistically significant decrease in erythropoietin dose in all treated patients. The present study as a pilot study suggests that the use of these two supplements in subsequent studies would not be associated with adverse effects on metabolic markers. Another limitation of this study was the research period. It is still hypothesized that different results would be achieved if the treatment is used for a more extended period (e.g., six months). Another significant limitation of the present study was that this study did not evaluate patients for possible differences among different races in Khuzestan province as previous studies have suggested that race can affect vitamin D levels in patients (29, 30).
5.1. Conclusions
Our study showed that the consumption of vitamin D supplements for three months can successfully compensate for vitamin D deficiency in HD patients and that it is not associated with adverse effects. D-Pearls were significantly more successful than calcium-D tablets in compensating for vitamin D deficiency. The findings also revealed an improvement in the patients’ hemoglobin levels during three months. However, the improvement was statistically significant only in the group taking calcium-D tablets. The interactions among PTH, calcium, and vitamin D seem to affect hematopoiesis and erythropoietin response and improve anemia effectively. Future studies could measure the effect of vitamin D in target communities by simultaneously controlling PTH-using drugs such as cinacalcet. Future researchers are recommended to further examine interactions among PTH, calcium, and vitamin D.