1. Background
Human immunodeficiency virus (HIV) infection varies from asymptomatic to severe immunodeficiency with secondary infections, malignancies, and other complications. According to a recent report on acquired immunodeficiency syndrome (AIDS)-info in 2019, the prevalence rate of new cases of HIV infection in Iran was estimated at 4100 (1200 - 12000) (1). Neurological disorders, such as peripheral nervous system (PNS) involvement, are common complications associated with HIV infection, occurring in both asymptomatic and symptomatic individuals (2, 3). The onset of symptoms may be acute, sub-acute, and chronic; also, depending on the type nerve of involved, motor, sensory, and autonomic neuropathy may exist (4, 5).
Neuropathy syndromes are almost related to the stage of the disease (6). Neuropathies may occur at all stages of HIV infection and cause significant disability. Although symptomatic neuropathy occurs in about 15 - 25% of HIV-seropositive patients, pathological evidence regarding peripheral neuropathy is found in almost all patients in the late stages of AIDS (7). The pathogenesis of neuropathy could be attributed to the effects of HIV-antigen (Ag), increased cytokines caused by infection, or antiretroviral-induced neurotoxicity (8). Distal sensory polyneuropathy (DSPN) is the most common neuropathy in HIV-seropositive patients, which is characterized by progressive symmetric paresthesia and painful lower extremity dysesthesia, seen in the mid and late stages of the disease (9). Other types of neuropathies in HIV-seropositive patients are acute inflammatory demyelinating polyneuropathy (AIDP), chronic inflammatory demyelinating polyneuropathy (CIDP), cytomegalovirus (CMV) neuropathy, drug-induced toxic neuropathy, vasculitis neuropathy, polyneuropathy multiplex, progressive polyneuropathy, and diffuse infiltrative lymphocytosis neuropathy (6).
2. Objectives
This study evaluates the frequency of different types of peripheral nerve involvement among HIV-seropositive patients visiting an HIV referral center, Ahvaz, Iran.
3. Patients and Methods
3.1. Study Design and Population
Fifty-nine HIV-seropositive patients visiting an HIV referral center in Ahvaz, Iran, over a 12-month period (from April 2018 to April 2019) were evaluated.
3.2. Inclusion Criteria
All HIV-seropositive patients referring to the HIV center, Ahvaz, Iran, during April 2018 to April 2019 were included in this study.
3.3. Exclusion Criteria
The patients who did not sign the informed consent form were excluded from the study.
3.4. Methods
All the participants signed the informed consent before enrolment. Data regarding age, sex, history of neuropathy, education, time of diagnosis and treatment, history of tuberculosis (TB), drug history (ART and others), and history of smoking were registered; also, data related to other causes of peripheral neuropathy, such as diabetes mellitus (DM), creatinine (Cr) level, and alcohol consumption were recorded, and the patients’ records were monitored. Physical exam, height measurement, and neurologic exam were performed. B12 level, viral load, and CD4 count were evaluated, and an electrodiagnostic study, known as nerve conduction velocity (NCV) test, was performed.
3.5. Ethical Considerations
Informed consent was obtained from each patient included in the study. The study protocol was approved by the local ethics committee and followed the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution's human research committee.
3.6. Statistical Analysis
The chi-square test was used to compare the nominal variables. The Kolmogorov-Smirnov test was used for evaluating the normal distribution of quantitative data, and the independent t-test was run to compare the quantitative variables. The collected data were analysed using SPSS version 22. Descriptive statistics such as frequencies, means, and standard deviation were reported for quantitative variables. Mann-Whitney test was applied to examine the differences in the mean age and height of these patients. Fisher’s exact test and chi-square test were used to investigate the relationship between neuropathy status and variables of gender, education, and smoking.
4. Results
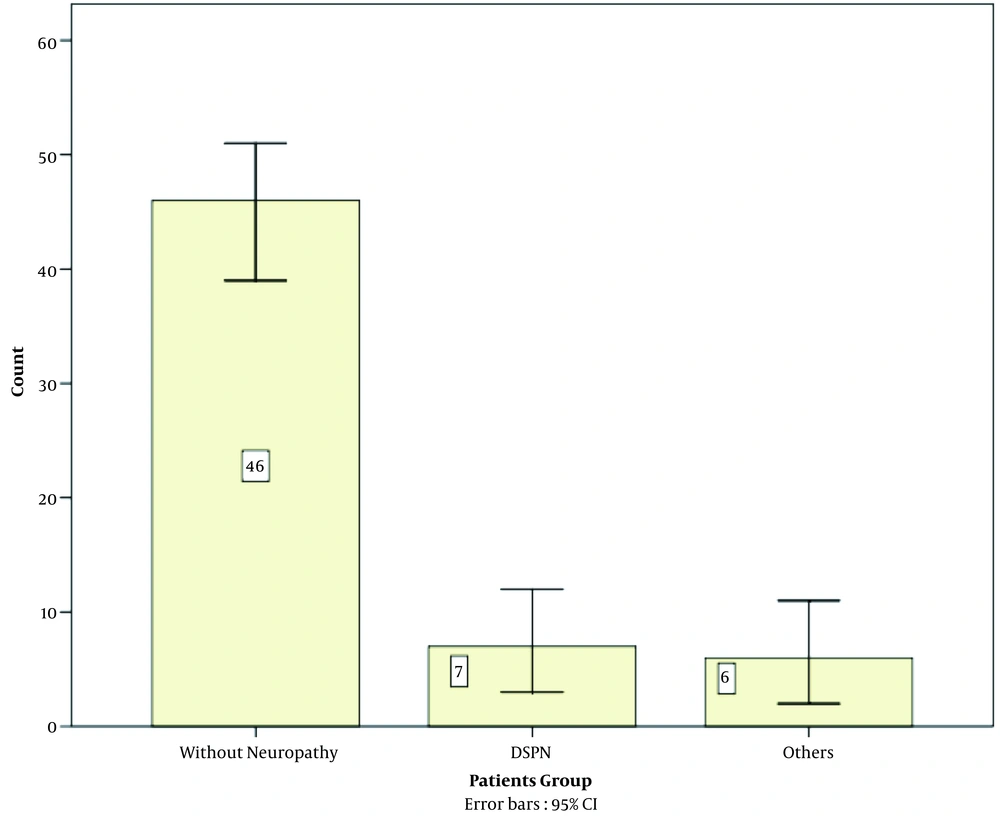
Of the 59 patients, 30 were male, and 29 were female, with a mean age of 37.88 years (Table 1). Thirteen (22%) patients had PNS involvement, 7 (11.9%) had DSPN, and 6 (10.2%) cases had other causes of PNS involvement (Figure 1), including two cases of carpal tunnel syndrome, one case of L5 radiculopathy, one case of motor neuropathy, and one case of CMV-induced radiculopathy. There was a significant relationship between age and PNS involvement in this study (P-value = 0.005). There was not any significant relationship between PNS involvement and gender, height, smoking, and education (Table 2). Other causes of PNS involvement, including creatinine and B12 level, were in a normal range, and none of the patients had DM and TB or were alcoholic (Table 1).
| Variables | Total (N = 59) |
|---|---|
| Gender | |
| Male | 30 (50.8) |
| Female | 29 (49.2) |
| Age | 37.88 ± 9.68 |
| Height | 168.17 ± 7.405 |
| Education | |
| Illiterate | 8 (13.6) |
| Preliminary | 50 (84.7) |
| Academic | 1 (1.7) |
| History of smoking | |
| Yes | 29 (49.2) |
| No | 30 (50.8) |
| History of alcohol use | |
| Yes | 0 |
| No | 59 (100) |
| Tuberculosis | |
| Yes | 0 |
| No | 59 (100) |
| Diabetes mellitus | |
| Yes | 0 |
| No | 59 (100) |
| Vit B12 deficiency | |
| Yes | 0 |
| No | 59 (100) |
a Values are expressed as No. (%) or mean ± SD.
| Variables | Total (N = 59) | With PNS (N = 13) | Without PNS (N = 46) | P-Value |
|---|---|---|---|---|
| Gender | 0.807 | |||
| Male | 30 (50.8) | 7 (53.8) | 23 (50) | |
| Female | 29 (49.2) | 6 (46.2) | 23 (50) | |
| Age | 37.88 ± 9.68 | 43.46 ± 6.09 | 36.30 ± 9.97 | 0.005 |
| Height | 168.17 ± 7.405 | 168.23 ± 6.76 | 168.15 ± 7.64 | 0.898 |
| History of smoking | 0.312 | |||
| Yes | 29 (49.2) | 5 (38.5) | 25 (54.3) | |
| No | 30 (50.8) | 8 (61.5) | 21 (45.7) | |
| Education | 0.111 | |||
| Illiterate | 8 (13.6) | 4 (30.8) | 4 (8.7) | |
| Preliminary | 50 (84.7) | 9 (69.2) | 41 (89.1) | |
| Academic | 1 (1.7) | 0 (0.00) | 1 (2.2) |
Abbreviation: PNS, peripheral nervous system.
a Values are expressed as No. (%) or mean ± SD.
Mean viral load in all the patients, cases with DSPN, and other cases of PNS involvement was 79838 (IU/mL), 610 (IU/mL), and 166709 (IU/mL), respectively. There was no significant relationship between PNS involvement and viral load (P-value = 0.579; Table 3). The mean diagnosis duration in patients without neuropathy, in cases of DSPN, and in cases with other causes of PNS involvement was 425, 454, and 397 days, respectively. There was no significant relationship between PNS involvement and the mean of diagnosis time (P = 0.913; Table 3). Also, the mean treatment duration for patients without neuropathy, for cases of DSPN, and for other causes of PNS involvement was 377, 369, and 317 days, respectively. There was no significant relationship between PNS involvement and the mean treatment duration (P = 0.179; Table 3). Fifty-two (88.1%) patients were treated with vonavir, but there was no significant association between PNS involvement and vonavir (P = 0.611; Table 4). The results also showed that there was not any significant relationship between ART and PNS involvement in this study because neurotoxic antiretroviral drugs (stavudine, zidovudine, and zalcitabine) were not used in these patients (P = 0.951; Table 4).
| Variable | Total (N = 59) | With PNS (N = 13) | Without PNS (N = 46) | P-Value |
|---|---|---|---|---|
| Serum level of CD4 | 601.19 ± 407.57 | 395.85 ± 214.51 | 659.22 ± 431.51 | 0.073 |
| Serum level of viral load (IU/mL) | 79838.05 ± 493890.81 | 77270.85 ± 277246.78 | 80563.57 ± 542122.01 | 0.579 |
| Diagnosis time (d) | 425.85 ± 90.90 | 427.31 ± 107.67 | 425.43 ± 86.94 | 0.913 |
| Treatment duration (d) | 370.17 ± 94.18 | 344.52 ± 76.77 | 377.39 ± 98.07 | 0.179 |
Abbreviation: PNS, peripheral nervous system.
a Values are expressed as mean ± SD.
| Variables | Total (N = 59) | With PNS (N = 13) | Without PNS (N = 46) | P-Value |
|---|---|---|---|---|
| Vonavir | 52 (88.1) | 11 (84.6) | 41 (89.1) | 0.611 |
| Kaletra2 & 3TC & TDF | 1 (1.7) | - | 1 (2.2) | - |
| Anzavir & Truvada | 1 (1.7) | - | 1 (2.2) | - |
| DTG & Truvada | 4 (6.8) | 2 (15.4) | 2 (4.3) | 0.579 |
| Kaletra & Truvada | 1 (1.7) | - | 1 (2.2) | - |
Abbreviations: PNS, peripheral nervous system; ART, antiretroviral therapy, 3TC, lamivudine; TDF, tenofovir disoproxil; DTG, dolutegravir; DSPN, distal sensory polyneuropathy.
a Values are expressed as No. (%).
5. Discussion
This study aimed to evaluate the frequency of different types of PNS involvement among HIV-seropositive patients. The results showed the frequency of PNS involvement among HIV-seropositive patients was 22%. This frequency was relatively lower than other similar reports (10-12). The prevalence of PNP in Zimbabwe and Brazil was 44 and 69.4% in individuals with HIV infection. Additionally, an important study was performed in 2021 to assess a neuro epidemiological screening tool in a rural Ugandan group with high HIV incidence. Amongst the selected individuals, 54% had a neurological abnormality, of which 46% were symptomatic (13). This may be due to the fact that the incidence of peripheral neuropathy increases with the progression of HIV and lower CD4-cell counts (< 100 Cells/µL) (14). Thus, they are more common in the later stages of HIV, whereas our cases were diagnosed in the last year and in the early stage of HIV. Moreover, our study demonstrated that the frequency of DSPN was relatively low (11.9%), but previous evidence revealed that DSPN is clinically present in 10 - 35% of HIV-seropositive patients without recognizing causes for their neuropathy (15-18).
In the present study, there was a significant relationship between PNS involvement and age. In 2019, a similar study by Puplampu showed that neuropathy increased with age (19). In the same year, a follow-up study in Ethiopia demonstrated that neuropathy increased in 40-year-old HIV-seropositive patients (20). Moreover, in 2017, the results of a study conducted in Brazil confirmed the relationship between neuropathy and age (21). In the present study, there was no significant relationship between PNS involvement and gender. However, a similar study by Saylor et al. from Uganda in 2017 found that female patients were more prone to neuropathy (22). There was no significant association between height and PNS involvement. Nevertheless, similar studies by Puplampu et al. in Ghana and Adem et al. in Ethiopia in 2019 demonstrated neuropathy could be increased in association with height (19, 20). There was no significant association between smoking and peripheral neuropathy in our study. In 2017, similar studies in Uganda and Brazil reported that neuropathy increased in smoking patients (20, 21). In this study, there was no significant relationship between education and PNS involvement. In disagreement with our findings, the study by Puplampu et al. demonstrated the incidence of neuropathy decreased with education (18, 19). In this study, no significant relationship was found between CD4 count and PNS involvement. However, the study by Puplampu et al. showed that neuropathy increased with increasing CD4 count.
In the present study, no significant relationship was found between viral load count and PNS involvement. In this context, de Almeida et al. and Wang et al. also showed that viral load count is independent of PNS involvement (23, 24).
In our study, no significant relationship was found between time of diagnosis and treatment duration and PNS involvement. There was no significant relationship between PNS involvement and the mean duration of diagnosis in the present study, because the recent study was performed among patients diagnosed in 2018, and more accurate results may be provided over a wide period of time (25). PNS involvement may be caused by the virus itself and its antibody production directly or indirectly, as well as drug-induced neurotoxicity (26-29).
5.1. Limitations
There are some limitations in the present study, including its observational nature with the potential for selection bias as our analyses were limited to individuals who were capable of returning for follow-up visits. Besides, the assessed relationship with factors subject to self-selection must be interpreted with caution. Due to limited information, we could not estimate some important variables, such as patients' ethnicity and the correlation between peripheral neuropathy and mean HIV onset time.
5.2. Conclusions
Peripheral neuropathy is relatively common in HIV-seropositive patients, the risk of which increases in patients with advancing age. Older age significantly upturns the risk of neuropathy. There was no significant relationship between PNS involvement and gender, height, smoking, education, CD4 count, viral load count, and the mean duration of diagnosis in the present study. Further studies with larger cohorts of patients are recommended.