1. Background
Evaluation of SLE patients by two-dimensional speckle tracking echocardiography at the time of diagnosis is necessary for the early diagnosis and prevention of cardiac involvement.
Detection of subclinical LV systolic dysfunction by GLS is possible in SLE patients despite normal LVEF in traditional echocardiography.
Systemic lupus erythematosus (SLE) is an autoimmune inflammatory syndrome of unknown etiology associated with multiple organ involvement, with significant differences in clinical prognosis (1). Various etiologies, such as genetic background, hormonal factors, and environmental variables, are introduced to activate the underlying chronic inflammatory cascade (2, 3). The SLE occurs in 10 - 50 cases per 100,000 of the general population. It affects women more than men (nine times more often) and occurs mainly in the second and third decades of life (4). Although rare, cardiac involvement could be the first clinical manifestation of SLE in 50% of patients, associated with high mortality due to pericarditis (5), myocarditis, Libman-Sacks endocarditis, pulmonary artery hypertension, and coronary heart disease. In 1560, William Osler described the cardiac manifestation of SLE. Libman and Sacks later described non-infectious SLE-induced endocarditis for the first time (6-9).
The prevalence of SLE cardiac complications depends on the study population, patient follow-up, and diagnostic techniques. Pericarditis or pleural effusion, valvular heart disease, myocarditis, systemic hypertension, heart failure, ischemic cardiac disease, and pulmonary hypertension have been reported in 11 - 58%, 6 - 84%, 5 - 75%, 22 - 69%, 7 - 44%, 5 - 16%, and 9 - 43% of cases, respectively (10-15). Histopathological studies on the autopsy series of SLE indicated myocarditis in about 40 - 78% of patients (16). However, it is less prominent in clinical practice, probably due to the slow myocardial inflammatory process in SLE. In the absence of cardiac symptoms, the exacerbation of left ventricular (LV) dysfunction occurs quite subclinically (17). Nonetheless, clinical myocarditis affects the survival of patients to a great extent. Diastolic dysfunction occurs prior to systolic dysfunction. Therefore, diastolic cardiac dysfunction is the primary manifestation of SLE myocardial involvement (18). Studies have also shown that the longer duration of the disease is coupled with more severe ventricular diastolic dysfunction (19). However, conventional echocardiography has not been able to detect early cardiac involvement in many situations, and there is a need for newer diagnostic modalities for better risk assessment of SLE patients.
However, subclinical ventricular dysfunction in SLE has not been well elucidated to date. Consequently, in the present study, we sought to evaluate subclinical LV dysfunction in patients with SLE with newer imaging modalities, such as global longitudinal strain (GLS) and three-dimensional echocardiography. This would probably help the early detection and treatment of cardiac involvement in SLE patients to improve their clinical course of the disease.
2. Methods
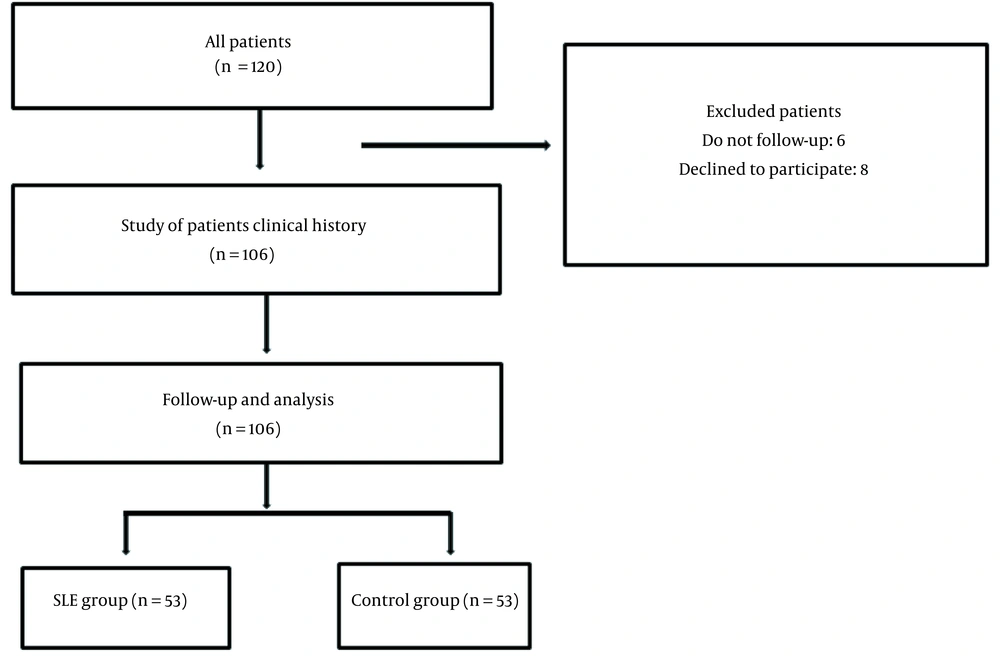
This prospective case-control study was conducted on a case group consisting of patients with SLE based on the diagnostic criteria of the American College of Rheumatology (ACR) (20), who referred to the rheumatology clinic of Rasoul Akram Hospital during 2018 and 2019. Patients in the case group did not have any history of heart diseases. The control group included age-and gender-matched healthy individuals without cardiac manifestations, chronic diseases, chronic inflammatory diseases, autoimmune diseases. In addition, the participants in the control group did not take any medications that affect lipid metabolism. The control group voluntaries were selected out of the hospital staff. The inclusion criteria were being an SLE patient without any previous history of heart disease, willingness to participate in the study, and fulfilling the ACR criteria for SLE disease. The exclusion criteria entailed a previous history of diseases, such as heart disease and dissatisfaction with participation in the study. Randomization was not performed, and convenient sampling was used for allocation (Figure 1).
All the study participants were evaluated by transthoracic echocardiography, Doppler echo, and tissue Doppler imaging (TDI). Accordingly, ventricular dimensions and LV ejection fraction (LVEF) were evaluated. Furthermore, E velocity (i.e., peak velocity blood flow from LV relaxation in early diastole), A velocity (i.e., peak velocity flow in late diastole caused by atrial contraction), E/A ratio, and E/e’ ratio was measured in both groups. The LV GLS index was also checked by two-dimensional (2D) speckle tracking echocardiography (STE) in all individuals. In addition, demographic characteristics, past medical history, and medicine consumption history were recorded in a special researcher-made questionnaire.
Examinations were performed using EPIQ 7 (Philips-Germany) echocardiography machine and Philips X5-1 xMATRIX array transducer for 2D echocardiography. Three apical views (apical four, three, and two chambers) were utilized to measure GLS. Moreover, automated cardiac motion quantification (aCMQ) was applied for offline strain analysis by QLAB software mac OS (USA) version 10.8.5.
2.1. Ethical Issues
All the study steps were performed according to the Helsinki Declaration. Written informed consent was obtained from all patients before enrolment. Patients were free to leave the study at any step without affecting their standard routine treatment. The data were used without disclosing the identity of the participants. The study protocol was approved by the Ethics Committee of Iran University of Medical Sciences (IR.IUMS.FMD.REC.1399.622).
2.2. Data Analysis
Data were analyzed by the SPSS software version 20 (SPSS Inc. Chicago, Il, The USA). Mean and standard deviation were used to describe quantitative descriptive data, and frequency and percentage were applied for qualitative variables. Kolmogorov-Smirnov test was utilized to evaluate the normal distribution of data. Independent t-test, one-way analysis of variance, and chi-square test or their non-parametric counterparts, such as the Mann-Whitney, Kruskal-Wallis, and Fisher's exact tests were used. P-value < 0.05 was considered statistically significant.
3. Results
The mean ± SD age of participants was 36.52 ± 11.95 years with a range of 16 - 75 years. Overall, 78.3% (n = 83) of the individuals were female, and 21.7% (n = 23) were male. The mean ± SD duration of SLE in the case group was 7.49 ± 5.62 years with a range of 0.5 - 25 years. The mean ± SD LVEF in all participants was 56.42 ± 3.24% (45 - 60%). In the test group, the prevalence of cardiovascular risk factors, proteinuria, hematological complications, skin complications, and glomerular filtration rate (GFR) reduction (less than 60 mL/min/1.73 m2) was 20.8% (n = 11), 45.3% (n = 24), 41.5% (n = 22), 79.2% (n = 42), and 15.1% (n = 8), respectively. There were no cerebral complications in the SLE patients in this study. Moreover, the antinuclear antibody (ANA) test and anti-dsDNA were positive in 84.9% (n = 45) and 45.3% (n = 24) of the SLE patients, respectively.
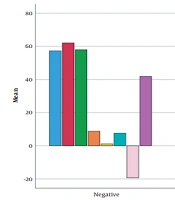
There were no significant differences between the two study groups in terms of mean age (Mann-Whitney, P = 0.221) or gender distribution (P = 0.339). Furthermore, E velocity, E/e' ratio, and GLS indices were significantly different between the two groups, while there were no statistically significant differences in other echocardiography indices. More detailed information is presented in Table 1.
| Variable | SLE Group (n = 53) | Control Group (n = 53) | P-Value |
|---|---|---|---|
| Age | 41.5 ± 14.09 | 38.38 ± 8.35 | 0.39a |
| Gender | 0.339b | ||
| Male | 2 | 4 | |
| Female | 51 | 49 | |
| LVEF (%) | 55.92 ± 3.81 | 56.98 ± 2.46 | 0.245c |
| E velocity (cm/s) | 60.37 ± 14 | 55.09 ± 10.67 | 0.021 |
| A velocity (cm/s) | 57.92 ± 16.79 | 52.83 ± 10.62 | 0.174c |
| E’ velocity (cm/s) | 7.39 ± 1.88 | 7.49 ± 1.1 | 0.645c |
| E/A ratio | 1.12 ± 0.38 | 1.07 ± 0.23 | 0.647c |
| E/e’ ratio | 8.5 ± 2.22 | 7.58 ± 2.2 | 0.016c |
| GLS (%) | -18.39 ± 3.18 | -21.07 ± 1.71 | < 0.001c |
Demographic and Echocardiographic Indices in the Two Study Groups
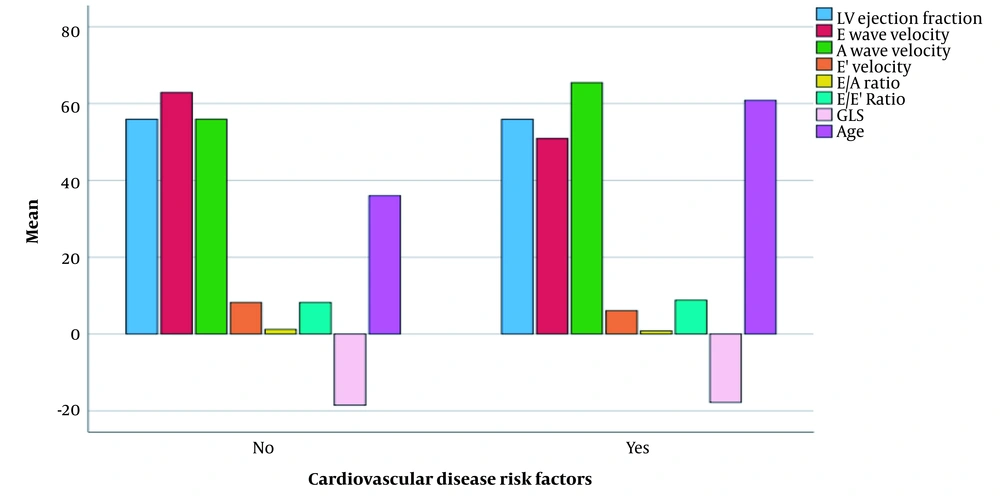
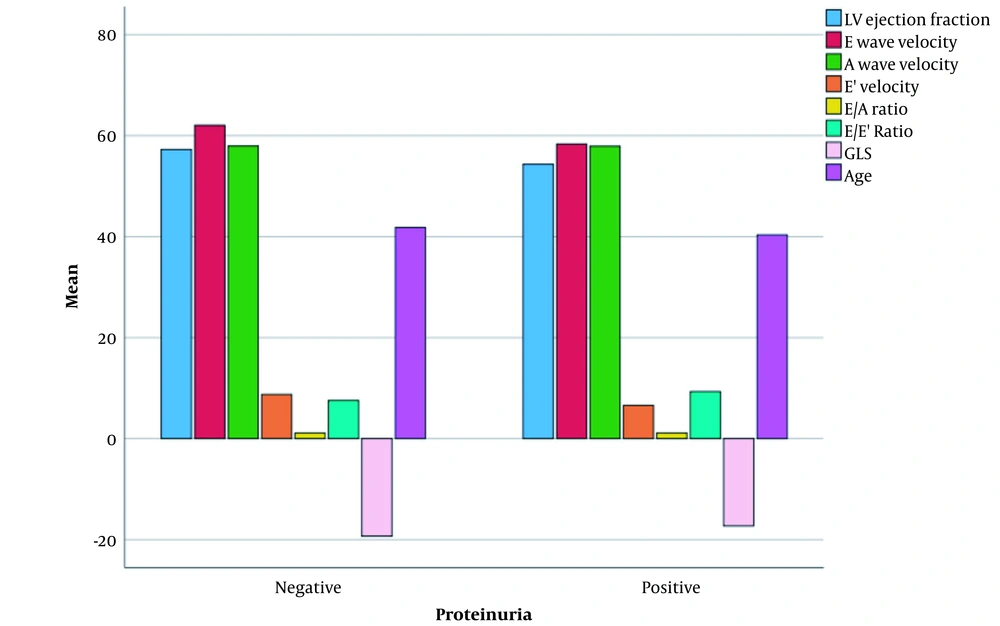
Mann-Whitney test revealed significant differences regarding E velocity, A velocity, e ' velocity, E/A ratio, and age between patients with and without cardiovascular risk factors in the case group (Figure 2). Furthermore, the result of the Mann-Whitney test showed that LVEF, e’ velocity and E/E' ratio were significantly different between patients with and without proteinuria in the case group (Figure 3).
Spearman’s correlation coefficient showed that the duration of SLE disease was significantly correlated with the E/A ratio (r = -0.287, P = 0.038) and GLS (r = -0.315, P = 0.021). On the other hand, the duration of SLE disease was not significantly correlated with LVEF, E velocity, A velocity, E’ velocity, and E/e’ ratio (P > 0.05). In addition, a significant correlation was found between age and A velocity (r = 0.406, P = 0.003), E/A ratio (r = -0.496, P < 0.001) and GLS (r = 0.229, P = 0.018). However, age did not have a significant correlation with LVEF, E velocity, E’ velocity, or E/e’ ratio (P > 0.05). Other findings of the present study showed that renal dysfunction and proteinuria or decreased GFR were correlated with both subclinical and LVEF systolic and diastolic dysfunction, while CAD (coronary artery disease) risk factors and hematologic complications in the SLE patients were correlated with diastolic dysfunction.
4. Discussion
In our study, there was no difference between the groups in terms of LVEF in the traditional echocardiographic examination. However, based on common LV systolic function parameters, such as LVEF, a low percentage of patients in the SLE group (9.4%) showed mild LV systolic dysfunction. In contrast, GLS revealed that the mean of this index (-18.39) was significantly lower in the SLE group than in the control group. We observed that GLS was significantly impaired in a large percentage of patients (about 40%) in the SLE group (considering the normal values of -18% to -21% suggested by the current recommendations (21). Therefore, these findings indicate primary and subclinical myocardial involvement due to SLE.
Buss et al. performed a study on 67 young patients with SLE without the usual symptoms of heart failure or angina. In the latter research, despite normal findings in standard 2D echocardiography, reduced LV systolic and diastolic myocardial flow was recorded by GLS (22). The results of Deng et al. showed that myocardial damage and LV regeneration still occur in SLE patients, even with normal LVEF (23). Similar findings were obtained in a study by Huang et al. on 34 patients with SLE and 34 healthy individuals, in which all strain components significantly diminished in the SLE patients. The authors recommended examining GLS as a simple method for identifying primary abnormalities in patients with SLE who may have normal LV systolic function in 2D echocardiography (24).
Moreover, in our study, lower values of E velocity were observed without difference in A velocity and the E/A ratio in the SLE patients compared to healthy controls. Since diastolic dysfunction had not yet occurred, the characteristics of diastolic dysfunction, including low velocity E, high velocity A, and the inverse E/A ratio, were not observed in the SLE patients, which is contrary to previous studies (18, 25, 26). In the investigation by Chung et al. (27), the LVEFs of SLE patients and healthy individuals were similar, while the E/A ratio in the SLE patients was much lower than the control group. In the present study, the E/A ratio was higher in the SLE group than healthy individuals without any significant difference. Reversing the E/A ratio from more than one to less than one is specific for diastolic dysfunction. However, the false normalization of the E/A ratio might occur in patients with severe diastolic dysfunction.
Many recent studies have demonstrated that new imaging modalities, such as TDI and cardiovascular magnetic resonance imaging (CMR), are more sensitive for the diagnosis of primary LV dysfunction in SLE patients. Teixeira et al. found no difference in LVEF and mitral E/A ratio between patients with SLE and controls. Instead, LV diastolic dysfunction was characterized by mitral annular tissue Doppler and mitral flow diffusion velocity (28). In addition, Puntmann et al. detected subclinical involvement of the myocardium and pericardium using CMR imaging (29).
Furthermore, the E/e' ratio has been shown to be the main indicator of diastolic function (30). In the current study, the E/e' ratio was significantly higher in the SLE group than the healthy participants. This result suggests that diastolic dysfunction has occurred even in patients with normal EF. The frequency of LV diastolic dysfunction increases with age (23). In our study, given the similarity of the mean age in the two groups of SLE and healthy controls, diastolic dysfunction associated with increased E/e’ can be attributed to the course of SLE disease.
We found no difference in echocardiographic indices between patients with positive or negative ANA tests. In contrast, in the SLE patients with positive anti-dsDNA tests, mean age and LVEF were significantly lower. Moreover, there was no difference in echocardiographic indices between female and male SLE patients. The disease duration was indicated to have a significant inverse correlation with the E/A ratio. In addition, the mean age was inversely and directly correlated with the E/A ratio index and A velocity index, respectively. Anti-dsDNA positivity in the SLE patients might be correlated with the severity of systolic cardiac dysfunction and a further decrease in LVEF. Noteworthy, none of the echocardiographic indices, except E/A and GLS, were correlated with SLE duration in our trial. The E/A index decreased with age in our study; therefore, it can be concluded that the reduced E/A in patients with long-term SLE is more probably related to aging and not the disease chronicity.
The mechanism of cardiovascular events in patients with SLE is largely unknown and may be multifactorial. However, some studies have shown that longer disease duration is correlated with more severe systolic and ventricular diastolic dysfunction (19, 31). Furthermore, some other systematic studies and meta-analyses showed that disease severity, together with disease duration, has a small prognostic effect on cardiovascular events (13, 32, 33). Consistent with the results of these studies, the lack of a correlation between LVEF and GLS in our study suggests that subclinical myocardial abnormalities in SLE patients can occur at any stage of the disease. Consequently, the early diagnosis seems necessary in the early management of cardiac involvement.
This study had some limitations. Due to the Coronavirus disease 2019 pandemic, it was impossible to assess disease severity according to the SLEDAI criteria. Therefore, it was impossible to evaluate the correlation between disease severity and echocardiographic indices. However, patients were selected based on convenient sampling, which could be accompanied by a potential selection bias. We suggest performing larger clinical trials with longer follow-ups on patients at different stages of SLE to elucidate the pathophysiology of cardiac involvement better. Moreover, ethnic diversity might lead to controversial reports, which could be better examined by multi-center studies. In such circumstances, the generalizability of data would be more rational.
4.1. Conclusions
The overall results of this study showed that in patients with SLE, subclinical systolic dysfunction measured by LV GLS could be detected despite normal systolic function in routine echocardiographic examination and regardless of disease duration and associated complications. Therefore, evaluating SLE patients by 2D STE at any time of diagnosis is necessary for the early diagnosis and prevention of cardiac involvement. However, further research is necessary on the various aspects of cardiac involvement in lupus to determine the significance of GLS changes in autoimmune diseases, such as SLE.