1. Background
Coronary ectasia is a heart disease characterized by endothelial cells and arteries dysfunction. Statistics showed that about 635,000 patients in the United States are diagnosed with coronary ectasia each year (1), of whom about 280,000 cases relapse. The prevalence of cardiovascular disease (CVD) has increased in the world from 10% in 2000 to 50% in 2019 (2). However, the prevalence of ectasia varies between 1.5% and 5%, and its prevalence in Iran is about 4% (3). Although various factors have been shown to be involved in disease development, the main cause of the pathogenesis of ectasia has not been determined yet. Inflammation and stimulation of immune responses are involved in disease progression.
When inflammation occurs, platelets and immune cells are activated. Platelet activation leads to increased secretion of coagulation factors and coagulation cascades stimulation (4). In addition, endothelial cell damage due to inflammation or platelets stimulation cause and develop coronary ectasia. Endothelial cell dysfunction is another cause of ectasia (4, 5). Studies have shown that tissue factors expression on the surface of endothelial cells leads to increased stimulation of immune cells, as well as platelet activation. These events ultimately increase coagulation factors production, especially fibrinogen in ectasia patients. Fibrinogen production in patients not only increases the activation of coagulation cascades, but also increases the dysfunction of endothelial cells, which can lead to disease progression and reduce patient survival.
It has been found that inflammatory cytokines production, especially Interlukine-17A (IL-17A), not only stimulates immune cells but also increases differentiation of neutrophils from myeloid precursors; it ultimately increases inflammatory mediators (6, 7). Red distribution width (RDW) is another diagnostic index for diagnosing inflammation. Studies have shown that increasing RDW in ectasia patients can be associated with disease progression and decreased patient survival. So far, no study has simultaneously evaluated IL-17A, fibrinogen, and RDW in patients, and these factors have been studied separately in different studies.
2. Objectives
In this study, for the first time, we simultaneously evaluated these three factors and examined their relationship with clinical and laboratory processes.
3. Methods
3.1. Design
The sample of this cross-sectional study included 140 CAD patients who were candidates for coronary angiography to confirm or rule out coronary artery involvement. All patients were selected from patients referred to Shariati Hospital of Tehran University of Medical Sciences, Iran from from Jun 2020 to October 2021. The patients’ information, including gender, age, cardiovascular risk factors, and medications were collected from the patients’ medical history.
The inclusion criteria were patients who were candidates for angiography, had no history of viral or microbial infections, and those whose information was complete. The exclusion criteria were as follows: patients with the history of recent infectious diseases (during past months), those with a history of chronic inflammatory or rheumatic diseases, those treated with anti-inflammatory drugs or corticosteroids in the last month, and those with a history of acute coronary syndrome or valvular or artificial valve diseases. All participants were selected randomly by available sampling method. After confirmation of their disease, an informed consent was obtained from all participants.
3.2. Sample
Using the following formula and considering the prevalence of 15% and the difference of 1.5 between the two groups, 140 people were selected as the sample size.
S = 2.5, d = 1.5, α = 0.05, β = 0.2
3.3. Instruments
Before angiography, fasting venous blood samples were taken, and serum IL-17A levels (a pro-inflammatory cytokine involved in inflammation reaction) were assessed by enzyme-linked immunosorbent assay (ELISA) kit (ab193732-IL-17 pig ELISA kit, Abcam, Cambridge, United Kingdom) in the hospital laboratory. Also, RDW (a method of blood counting that shows the size of red blood cells) was measured based on blood cell counting device (K-X-21N auto analyzer). Also, serum fibrinogen (one of the coagulation factors that is made by the liver and prevents bleeding by clot formation) levels were assessed by the clotting method of Clauss with Stago Compact Max (Diagnostica Stago, Asnieres, France).
3.4. Data Collection
Coronary ectasia is defined as abnormal dilatation of the artery (more than 1.5 times of normal size), and CAD is defined as the involvement of more than 50% in at least one of the epicardial arteries. Based on the severity of coronary artery involvement, patients were divided in two equal groups (n = 70 in each) of mild CAD (individuals with coronary artery involvement less than 50%) and multi-vessels CAD (patients with more than 50% stenosis in coronary arteries) according to the number of involved vessels (one, two, or three). The mean fibrinogen, RDW, and IL-17A levels were evaluated, and their relationship with patients’ previous history was examined.
This study was approved by the ethics committee of Tehran University of Medical Sciences (IR.TUMS.THC.REC.1399.050), Tehran, Iran. Also, an informed oral consent was obtained from each patient.
3.5. Data Analysis
The collected data were entered in SPSS version 22. Data distribution was examined by Kolmonogorov-Smirnov test. chi-square, independent t-test, and one-way analysis of variance (ANOVA) were used for data with normal distribution. In case of non-normal data, parametric tests were used.
4. Results
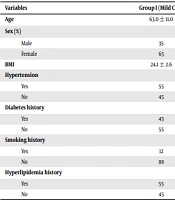
The patients’ demographic information is shown in Table 1. The mean body mass index (BMI) in the multi-vessels CAD group was significantly higher than the mild CAD group (P = 0.01). Moreover, the history of smoking (P = 0.04), hypertension (P = 0.01), hyperlipidemia (P = 0.03), and diabetes (P ≤ 0.001) in the multi-vessels CAD group was significantly higher than the mild CAD group (Table 1).
| Variables | Group I (Mild CAD) | Group II (Multi-Vessels CAD) | P-Value |
|---|---|---|---|
| Age | 63.0 ± 11.0 | 60.6 ± 12.1 | 0.08 |
| Sex (%) | 0.6 | ||
| Male | 35 | 28 | |
| Female | 65 | 72 | |
| BMI | 24.1 ± 2.6 | 28.1 ± 3.2 | 0.01 |
| Hypertension | 0.01 | ||
| Yes | 55 | 68 | |
| No | 45 | 32 | |
| Diabetes history | < 0.001 | ||
| Yes | 45 | 78 | |
| No | 55 | 22 | |
| Smoking history | 0.04 | ||
| Yes | 12 | 49 | |
| No | 88 | 51 | |
| Hyperlipidemia history | 0.03 | ||
| Yes | 55 | 85 | |
| No | 45 | 15 |
The total cholesterol levels in the mild and multi-vessels groups were 210.4 ± 32.0 and 250.7 ± 39.8, respectively, which was statistically significant (P-value = 0.03, OR = 3.2). The mean high-density lipoprotein (HDL) was 68.2 ± 14.2 in the mild group and 55.9 ± 11.2 in the multi-vessels group, which was also statistically significant (P-value = 0.02, OR = 4.1). Also, the mean low-density lipoproteins (LDL) in the mild group was 133.8 ± 31.5, and it was 167.8 ± 33.4 in the multi-vessels group, which was statistically significant (P-value = 0.04, OR = 2.5). TG level in the multi-vessels group was higher than the mild group (OR = 4.9) (Table 2). Also, as shown in Table 2, the odds ratio based on lipid profiles in the multi-vessels group was higher. The mean of all the three factors, including fibrinogen, IL-17A, and RDW was higher in the multi-vessels group, which was statistically significant (< 0.05). It was also shown that increasing the levels of RDW, IL-17A, and fibrinogen in the multi-vessels group increased the chance of ectasia compared to the mild group (Table 3).
| Variables | Group II (Multi-vessels CAD) | Group I (Mild CAD) | P-Value | OR |
|---|---|---|---|---|
| Total cholesterol | 210.4 ± 32.0 | 250.7 ± 39.8 | 0.03 | 3.2 |
| HDL | 68.2 ± 14.2 | 55.2 ± 11.2 | 0.02 | 4.1 |
| LDL | 133.8 ± 31.5 | 167.8 ± 33.4 | 0.04 | 2.5 |
| TG | 220.2 ± 41.3 | 250.8 ± 42.1 | 0.001 | 4.9 |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglyceride.
| Variables | Group II (Multi-vessels CAD) | Group I (Mild CAD) | P-Value | OR |
|---|---|---|---|---|
| Fibrinogen (mg/dL) | 305.4 ± 35.78 | 337.3 ± 75.48 | 0.01 | 1.3 |
| IL-17A (ng/dL) | 1.4 ± 0.5 | 4.86 ± 3.2 | 0.05 | 1.9 |
| RDW (%) | 12.3 ± 1.36 | 15.4 ± 1.5 | 0.03 | 1.2 |
In addition, the levels of fibrinogen (OR = 1.87), RDW (OR = 2.2), and IL-17A (OR = 1.61) were higher in patients with three involved vessels than in patients with two or one involved vessel(s), which was statistically different (P < 0.05). In other words, the more severe the coronary artery involvement in patients, the higher the mean of fibrinogen, RDW, and IL-17A (Table 4).
| Variables and Vessels Involved | Mean ± SD | P-Value | OR |
|---|---|---|---|
| Fibrinogen (mg/dL) | 0.003 | 1.87 | |
| One | 325.5 ±16.6 | ||
| Two | 310.5 ±17.5 | ||
| Three | 245.3 ±32.5 | ||
| IL-17A (ng/dL) | 0.001 | 1.61 | |
| One | 1.6 ± 0.23 | ||
| Two | 1.8 ± 1.2 | ||
| Three | 2.5 ± 1.0 | ||
| RDW (%) | 0.01 | 2.2 | |
| One | 12.2 ± 1.01 | ||
| Two | 13.1 ± 1.4 | ||
| Three | 13.1 ± 1.4 |
5. Discussion
CAD is one of the most common diseases with increasing prevalence in the world. The death prevalence due to this disease is gradually increasing, which poses a special challenge for patients (8). Coronary artery ectasia is another common heart disease characterized by endothelial cells dysfunction. In ectasia, the diameter of the vessels becomes larger than normal. Studies have shown that inflammation and coagulation factors play an important role in the development of ectasia.
The etiology of CAD has not been determined yet; however, it has been shown that many environmental and genetic factors are involved in its pathogenesis (9). Since inflammation and stimulation of immune system are effective in disease pathogenesis, inflammatory cytokines increment, including interleukins 6, 1, and 17 in patients with CAD causes disease progression and worsens the clinical conditions of patients. In addition, increasing coagulation and blood factors in patients not only causes thrombosis, but also leads to increased inflammation and apoptosis of body cells.
The results of studies have shown that inflammatory cytokines increment leads to increased expression of tissue factors on the surface of macrophage cells, as well as other cells in the body (10), which leads to coagulation and stimulation of coagulation cascades. Ultimately, this process increases ccoagulation factors production, including fibrinogen. Increased fibrinogen not only stimulates platelets in patients and increases pro-coagulant factors production, but also stimulates immune system and chemotaxis of inflammatory cells to endothelial cells (11). This displacement leads to endothelial cells dysfunction, which eventually leads to CAD (12). In addition, it has been shown that increase in some laboratory indices can be a sign of inflammation and disease progression. One of these indices is RDW, which has been shown that its elevation can be consistent with disease progression. Also, it has been shown that some cytokines and coagulation factors increment can be a sign of increased disease progression and decreased patient survival (13). Therefore, in this study, we evaluated IL-17A, fibrinogen, and RDW in ectasia patients.
In the present study, the results showed that IL-17A levels were higher in patients with more than 50% coronary artery stenosis compared to patients with less than 50% CAD (mild CAD); but this difference was not statistically significant. On the other hand, it was shown that the mean of IL-17A was higher in patients with ectasia compared to patients without ectasia, which was statistically significant. It was also found that the odds ratio of ectasia was higher due to increased IL-17A in patients in the second group.
Uygun et al. evaluated IL-17A level and investigated its relationship with the incidence of coronary artery ectasia. In this study, 41 patients with coronary artery ectasia and 45 patients without coronary artery involvement were compared with angiography. IL-17A levels (assessed by ELISA) were significantly higher in the coronary artery ectasia group (4.86 ± 3.24 vs. 1.37 ± 1.56). No significant relationship was observed between IL-17A levels and the severity of coronary artery ectasia. Also, the level of fibrinogen and RDW in patients with ectasia was much higher than healthy controls. This lack of significant difference can be due to the low sample size (14).
Keser et al. (2016) evaluated the relationship between RDW and the severity of coronary ectasia. They included a total of 126 patients with coronary artery ectasia, 104 patients with coronary heart disease, and 76 patients with normal coronary status. The results showed that the RDW value for type 1 ectasia was much higher than other types of ectasia, which was consistent with our study (15). Regarding the mean RDW, the results showed that it was higher in patients with coronary artery stenosis than 50% compared to patients with less than 50% CAD (mild CAD); also, the mean RDW in patients with ectasia was higher compared to patients without it, which showed a statistically significant relationship. It was also found that the odds ratio of ectasia was higher due to increased RDW in patients in the second group.
In a study by Eriksson et al., the value of serum fibrinogen levels was examined to predict CAD in women. In this regard, two groups of patients with acute coronary syndrome and healthy controls were included. The fibrinogen levels in women with the acute coronary syndrome were much higher than in healthy controls. After adjusting heart disease risk factors, serum fibrinogen levels were still reported with OR = 3 in women with coronary artery involvement beyond healthy control women. The results of this study were similar to our study (16).
Lima et al. assessed the relationship between fibrinogen levels and the severity of coronary artery involvement. Blood samples were taken from 17 patients with normal CAD, 12 patients with mild or moderate atheromatosis, and 28 patients with severe atheromatosis; fibrinogen level was determined by coagulometric method. In this study, serum fibrinogen levels in patients with severe atheromatosis were much higher than the other two other groups. There was also a significant relationship between fibrinogen levels and the severity of coronary artery involvement (correlation coefficient equal to 0.50). The results of this study were similar to our study (17).
Also, Özde et al. showed that fibrinogen increment in ectasia patients was significantly higher than the control group. The results also showed that measuring fibrinogen levels in ectasia patients can be effective in monitoring and treating the disease. The results of this study were similar to our study (18). Consistent with these results, findings of the present study showed that fibrinogen levels in patients with ectasia and coronary artery stenosis greater than 50% was higher compared to patients without ectasia and CAD less than 50% (mild CAD), respectively; this difference was statistically significant.
5.1. Limitations
The low sample size was one of the main limitations of this study. Also, we failed to perform a follow-up to evaluate the changes in variables after the treatment due to time restrictions.
5.2. Conclusion
Our results showed that IL-17A, fibrinogen, and RDW levels in the patients with ectasia and people with coronary artery stenosis more than 50% were higher than patients without coronary ectasia and mild CAD (coronary artery stenosis less than 50%), respectively. Thus, evaluation of inflammatory factors such as IL-17A, laboratory indices such as RDW, and coagulation factors such as fibrinogen in ectasia and CAD patients can be helpful. Evaluating these factors in terms of measurement time, as well as price, is more cost-effective than other methods. Measurements of these variables are also available in many regions.
Further studies with larger sample sizes are required. Also, it is recommended to evaluate the changes of IL-17A, RDW, and fibrinogen levels according to the type of study. It is better to compare the levels of RDW, IL-17A, and fibrinogen with those of normal individuals.