1. Context
Coronary artery ectasia (CAE) or aneurysm is a type of heart disorder characterized by localized or diffuse non-obstructive arteries. Chronic disease is one of the prominent features of the disease that can affect the treatment and clinical course of patients (1). Besides, CAE is an angiographic finding in 1 - 5% of patients with coronary artery disease (CAD), which is more common in men (2, 3). Although the exact pathophysiology of CAE is unclear, atherosclerosis is thought to be responsible for more than 50% of CAE cases; it is the most common cause, followed by congenital and acquired factors (4, 5). Also, CAE may occur during atherosclerosis development. There are other theories that CAE may also occur in the coronary and other vascular systems, so it may be independent of atherosclerosis (6). Moreover, CAE can be associated with many factors such as increased inflammatory responses, impaired immune function, and endothelial dysfunction (7). An imbalance in the production of inflammatory factors and increased secretion of cytokines are associated with coagulation development and impaired immune cells function, both of which are considered essential factors in the pathogenesis of vascular disease (8).
Anti-inflammatory drugs may help control the coagulation system and the clinical condition of CAE patients. In the present study, we aimed to investigate the possible responses of patients to anti-inflammatory and anticoagulation drugs and various theories about the inflammatory and coagulation mechanisms in CAE patients.
2. Classification of Ectasia
Ectasia is divided into several groups based on the number of vessels involved. In the first type, ectasia usually involves two or three blood vessels. In the second type, ectasia is usually diffuse in one vessel and localized in the other. In other types of ectasia, diffuse or localized ectasia is usually seen alone (8).
3. Anti-inflammatory Drugs
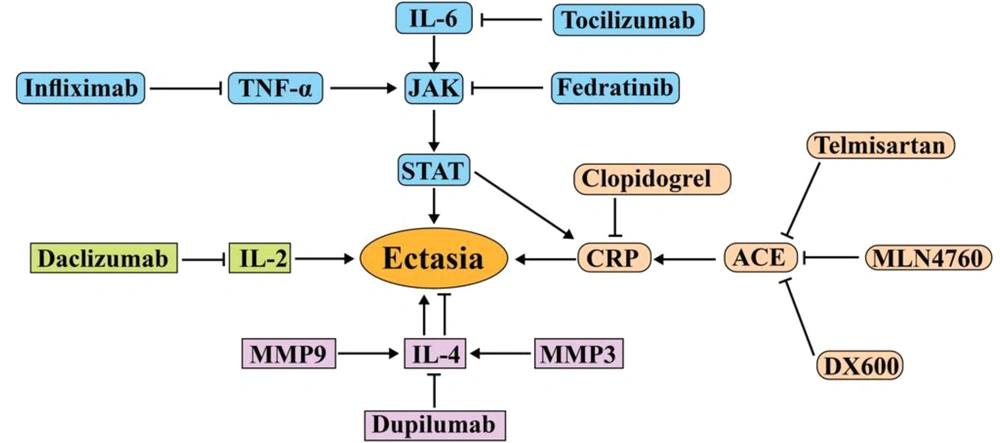
Inflammation is a vital process for CAD and atherosclerosis (9). Increased inflammatory mediators such as interleukins, C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) are considered risk factors for CAD, which are associated with increased morbidity and mortality (10, 11). The increasing number of neutrophils, monocytes, and lymphocytes as cells producing inflammatory factors in CAE patients and markers derived from dendritic cells in ectasia patients with CAD indicates the role of inflammation in CAE patients (Table 1) (2, 12). IL-6 secreted by monocytes can impair the function of the coagulation system and inflammation by increasing procoagulant factors and decreasing anticoagulants (13). IL-6 not only can play a role in inflammatory responses alone but also can be a potent stimulus for the production of other inflammatory mediators such as CRP and tumor necrosis factor-α (TNF-α) (14). According to these findings, the administration of IL-6 and JAK2-STAT3 inhibitors, along with cardiac drugs, can help CAE patients recover (Figure 1). Tocilizumab and sarilumab are IL-6 inhibitors that bind to IL-6 receptors to block its inflammatory activity and effects (15, 16). Tocilizumab acts like a double-edged sword, as it can lead to the development of aneurysms (17). It increases adverse blood fats and cholesterol, which are important risk factors for heart disease (Figure 1) (18).
| Medicines | Mechanism | Outcome | References |
|---|---|---|---|
| Tocilizumab and sarilumab | Anti-IL-6 receptor antibody | Increasing undesirable fats and cholesterol and coronary artery aneurysm development; directly affecting the inflammatory condition and, therefore, indirectly controlling the clotting system | (16, 19) |
| Infliximab and etanercept | TNF-α inhibitor | Reducing CRP, ESR, fibrinogen, and arterial aneurysms; decreasing platelet count and reactivity; increasing MPV | (20, 21) |
| Fedratinib | JAK2 dedicated inhibitor | Inhibiting JAK2, a critical mediator in signaling inflammation, and thus reducing the effects of increased inflammatory cytokines | (22, 23) |
| Daclizumab and basiliximab | Anti-IL-2 receptor | Improving vascular endothelial function in postoperative stent placement | (24) |
| Dupilumab | IL-4 receptor inhibitor monoclonal antibody | Reducing the risk of atherosclerosis by preventing inflammatory risk factors; inhibiting IL-4 as an anti-inflammatory cytokine and thus leading to thrombosis and coagulation promotion | (25, 26) |
| Secukinumab | IL-17 inhibitor | Improving endothelial function | (27) |
| Anakinra | IL-1 receptor antagonist | Decreasing the levels of ferritin, fibrinogen, and CRP; preventing macrophage activation syndrome and reducing atherosclerosis immune-inflammatory response | (28) |
| Lipoxins | Binding to its receptors (ALX and GPR32) on human endothelial cells | Stimulating the endothelial production of vasoprotective and antithrombotic mediators; genetic deletion of lipoxin receptor causes aortic dilation induced by angiotensin II infusion, decreases vascular collagen, and increases inflammation. | (29) |
Abbreviations: IL, interleukin; TNF-α, tumor necrosis factor-alpha; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; MPV, mean platelet volume; JAK2, Janus kinase.
Molecular pathways involved in the pathogenesis of ACE and the drugs used to treat them. Tocilizumab and fedratinib prevent inflammation and progression of ACE by targeting IL-6 and JAK, respectively. MLN4760 and DX600 also prevent the progression of ACE by inhibiting the angiotensin converter. Dupilumab can be effective in treating ectasia by targeting IL-4. Daclizumab can also be effective in treating ectasia by targeting IL-2. Abbreviation: JAK, Janus kinase; ACE, angiotensin-converting enzyme; CRP, C-reactive protein; TNF-α, tumor necrosis factor-α.
Given that CAE is six times more prevalent in patients with familial hypercholesterolemia than in healthy individuals, it can be hypothesized that tocilizumab use may be associated with increased cholesterol in CAE patients (30). However, it can control IL-6-related inflammatory responses and their destructive effects on the coagulation system by directly affecting inflammatory conditions; thus, it effectively reduces the risk of CAE. Side treatment of CAE patients with tocilizumab is an important and controversial issue; its use does not seem effective in this group of patients (Table 1). To control the effects of tocilizumab, it can be used along with lipid-lowering drugs. Alirocumab is a human monoclonal antibody against proprotein convertase subtilisin-kexin type 9 (PCSK9) that helps the liver reduce the level of bad cholesterol in the blood (31). It is used to treat atherosclerosis and prevent fat deposition in the arteries’ walls (32). Thus, it can be hypothesized that CAE patients with high blood lipids, which need anti-inflammatory drugs to control the coagulation system, can use lipid-controlling drugs along with cytokine inhibitors.
Fedratinib, as a specific inhibitor of JAK2, can block the activity of many inflammatory factors that are affected by this receptor or activated by IL-6, IL-17, and IL-22 (22). Also, IL-6 activates STAT3 by activating JAK2 and JAK1, which, in turn, induce Th17 differentiation and the secretion of inflammatory cytokines from this cell (33). Therefore, JAK inhibitors such as fedratinib can lead to the disconnection of IL-6 signaling with Th17 and the coagulation system. It seems that JAK2 inhibition, as one of the main factors involved in the inflammatory process, can prevent an overactive coagulation system (Figure 1).
Production of TNF-α from monocytes, macrophages, and lymphocytes, which are enlarged cells in CAE patients, has destructive effects on endothelial cells (ECs) (34). Besides, TNF-α plays a vital role in the pathogenesis of CAE by increasing the expression of adhesion molecules and altering vascular permeability (35). Coronary circulation involvement occurs following vascular injuries in CAE patients, which slows and disrupts blood circulation in these patients (36). In fact, the dysfunction of ECs by inflammatory mediators causes more inflammatory responses and worsens the patient’s clinical condition (30). As a result, blocking TNF-α and using antibodies against it may effectively control inflammation in CAE patients.
Infliximab and etanercept as TNF-α inhibitors can reduce CRP, ESR, and fibrinogen levels and prevent the coagulation system from becoming overactive in CAE patients (37, 38) (Table 1). Studies have shown that etanercept can cause hyperlipidemia (39), so its administration to CAE patients is controversial, and more studies are needed to reach a definitive conclusion about the effect of this drug on CAE patients’ blood lipids. Infliximab has a positive effect on the clinical condition of patients with arterial aneurysms, and its injection has reduced CRP (37). As high blood fats are a prognostic factor in coronary heart disease and the accumulation of lipoproteins triggers inflammatory immune responses and atherosclerotic plaques, it is possible to prevent etanercept-induced hyperlipidemia by ezetimibe. It is a potent inhibitor of sterol uptake and selectively inhibits the uptake of bile cholesterol and dietary cholesterol in the small intestine (Figure 2) (40, 41).
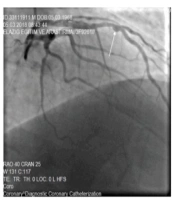
Ectasia involving vessels as diffuse and localized (42)
Based on the evidence, ezetimibe combined with statins is more effective in reducing inflammation. In addition to inhibiting 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, statins can lower low-density lipoprotein (LDL) cholesterol. It prevents fatty acid-related disorders and improves cardiovascular disease by reducing inflammation and resolving endothelial disorders (43-45).
Angiogenesis is a significant regulator of plaque growth, and statins can also inhibit angiogenesis. Statins also reduce the expression of cyclooxygenase-2 (CoX-2) and matrix metalloproteinase-9 (MMP-9) in vascular endothelium (46). As known, CoX is a pro-inflammatory enzyme with pro-angiogenic effects, and there is a functional relationship between CoX-2 activity and MMPs secretion (47, 48). Due to the properties of statins, it seems that prescribing this drug for CAE patients with hyperlipidemia can be effective in improving vascular endothelial function by inhibiting MMPs.
The imbalance between T-helper (Th) cells may lead to ectasia and atherosclerosis (49). Th1 cells are involved in the pathophysiology of heart disorders by secreting IL-2. Studies have shown that CAE patients have higher IL-2 levels than healthy individuals (50, 51). Another study found that IL-2 and IL-4 were significantly lower in CAE patients than in healthy individuals (52, 53). Secreted IL-4 by Th2 can have protective effects against vascular damage and be involved in vascular remodeling by inhibiting the production of inflammatory cytokines (54). Due to the protective and anti-inflammatory role of IL-4, its reduction in CAE patients is associated with a poor prognosis. The induction of anti-inflammatory pathways by IL-4 and suppression of monocytes indicate the atheroprotective effect of IL-4 (55, 56).
On the other hand, an increase in IL-10, an anti-inflammatory factor, has been reported in CAE patients (57). It seems that this increase in IL-10, along with an increase in inflammatory factors, could indicate a regulated immune response to the progression of inflammation, which can inhibit the activity of inflammatory cytokines by over-production of IL-10. An imbalance between the inflammatory factors produced by Th1 and the anti-inflammatory factors secreted by Th2 contributes to CAE and impaired vascular function.
Drugs such as daclizumab and basiliximab, which bind to IL-2 receptors on the surface of T cells, can inhibit IL-2 dysfunction. They eliminate the imbalance between Th1 and Th2 by increasing recombinant IL-10 and IL-4 or increasing Th2 expression (56, 58). No study has been performed on the effects of daclizumab and basiliximab on CAE patients. Further studies about these drugs and anti-inflammatory cytokines may speed up the treatment procedure for CAE patients.
Stimulated inflammatory responses may reflect the increased activity of MMPs. Destruction of the extracellular matrix by MMPs weakens the connective tissues of the vessel wall and makes them thinner and eventually dilated (59, 60). Studies have shown that MMP3 and MMP9 levels are high in CAE patients, and aneurysm development may be associated with increased MMP3 and MMP9. Besides, MMP3 hydrolyzes extracellular matrix compounds such as proteoglycans and collagens and can also activate MMP1 and MMP9 (61, 62).
One study showed that reducing MMP9 could prevent atherosclerotic damage and ectasia (63). Inactivation of MMP3 also reduces aneurysms (64). However, an increase in IL-4 is associated with an increase in MMP3 and MMP9 (48), so blocking the IL-4 receptor can reduce the risk of CAE (Table 1). In response to inflammation, IL-4 level increasedby the anti-inflammatory effects; on the other hand, its increase is associated with MMP3 and MMP9 elevation (65, 66). These two effects can be considered risk factors in CAE; it seems that the different functions of these cytokines are essential in CAE patients.
Dupilumab is a human monoclonal antibody that inhibits its signaling by binding to the IL-4 receptor and down-regulates inflammatory synthesis. It reduces the risk of atherosclerosis by inhibiting MMP12, IL-6, and Th2-related factors (25). On the other hand, by inhibiting IL-4 as an anti-inflammatory cytokine, this drug can lead to the development of thrombosis, coagulation, and even ischemic stroke (67). One study showed that IL-4 deficiency could lead to size reduction of vascular lesions in atherosclerosis (68). The multiple roles of IL-4 in the inflammatory and coagulation process and different outcomes with the use of its inhibitors are essential issues; further investigation and research are required to diagnose and treat CAE.
In general, reducing and controlling the level of MMPs can prevent damage to the extracellular matrix. Tissue inhibitor of MMPs (TIMPs) regulates the activity of MMPs, which inhibits and suppresses TIMPs by increasing inflammatory interleukins and MMPs (69). Thus, to improve the function of TIMPs in CAE patients and prevent vascular injury, reducing and inhibiting the production of mediators and inflammatory cells could be a way to treat and improve the clinical condition of CAE patients.
Inflammation is recognized as a significant component in the process of atherogenesis. Consequently, anti-inflammatory therapies can prevent the accumulation of inflammatory cells and vascular disorders in CAE patients. It should be noted that the administration of anti-inflammatory drugs to CAE patients requires the measurement of inflammatory cytokines in patients, followed by the use of an enhanced cytokine-associated inhibitor. The study of CAE patients regarding inflammatory factors and their treatment with anti-inflammatory drugs has not been discussed enough to provide a definitive theory in CAE patients. Future studies should pay attention to the role of anti-inflammatory drugs in these patients.
4. Anti-thrombotic Drugs
The imbalance between fibrinolytic systems and coagulation is associated with thrombosis and vascular disorders in CAE patients. The formation of thrombosis in ectatic vessels is one of the most important causes of mortality in patients with CAE (70, 71). It is not clear why CAE increases the risk of thrombosis, but studies have shown that fibrolytic system function is not normal in ectatic patients. The levels of α1-antitrypsin, α2-macroglobulin, and α2 plasmin inhibitor as plasmin inhibitors (one of the main factors in fibrinolysis) are increased in CAE patients (72). Increased plasmin inhibitors reduce the efficiency of the fibrinolysis system in CAE patients, which is associated with an increased risk of clinical coronary events (Table 2).
| Medicines | Mechanism | Outcome | References |
|---|---|---|---|
| Urokinase and streptokinase | Activation of plasminogen to plasmin | Decreasing fibrinogen levels; activating fibrinolysis to degenerate thrombosis (Excessive use can lead to bleeding) | (73, 74) |
| rt-PAS (alteplase, reteplase and tenecteplase) | Catalyzing the conversion of plasminogen to plasmin | Patency of blood vessels from coagulation lesions (Its short half-life may lead to the recurrence of blood clots) | (75, 76) |
| Aspirin | Irreversibly inhibiting cyclooxygenase | Elimination of thrombosis and improvement of patients with chronic treatment; slowing down the blood clotting process (High platelet aggregation with aspirin consumption increases myocardial infarction) | (77, 78) |
| Clopidogrel and ticlopidine | Platelet ADP receptor (P2Y12) antagonists | Increasing thrombolysis induced by streptokinase; causing anti-inflammatory effects; reducing the risk of thromboembolic attack (Individual platelet responses depend on the genetic, cellular, clinical, and environmental factors) | (79, 80) |
| Tirofiban and eptifibatide | gpIIb/IIIa receptor blockers | Improving endothelial dysfunction in different clinical situations. Reversal of endothelial dysfunction induced after PCI | (81, 82) |
| Dabigatran | Direct thrombin inhibitor | Attenuating endothelial dysfunction; decreasing expression of inflammatory molecules; preventing the development of atherosclerosis; preventing systemic thromboembolic events in patients with atrial fibrillation | (83) |
Abbreviations: rt-PAS, recombinant tissue plasminogen activators; ADP, adenosine diphosphate; gpIIb/IIIa, glycoprotein IIb/IIIa; PCI, percutaneous coronary intervention.
Urokinase and streptokinase, two anticoagulant enzymes, have been shown to reduce the risk of heart attack and mortality (73). By saturating the α2-antiplasmin binding site, these two enzymes cause hyperplasminemia and increase fibrinogen degradation (74). It should be noted that these two intracoronary thrombolytic drugs increase the risk of postoperative bleeding in patients with fibrinogen levels less than 100 mg/dL (84). A study compared the urokinase and streptokinase and showed that postoperative bleeding was higher in patients receiving streptokinase than in those receiving urokinase. Unlike streptokinase, urokinase is not antigenic, and antibody-mediated resistance does not occur with this enzyme.
Like urokinase, recombinant tissue plasminogen activator (rt-PA) has no antigenic or pyrogenic properties and produces much less systemic fibrinolysis than streptokinase (85, 86). However, the short half-life of rt-PA may lead to recurrence and early clotting of arteries. Therefore, it seems that urokinase could be a better anticoagulation drug for treating vascular lesions due to increased coagulation in CAE patients. It is recommended that CAE patients be treated with anticoagulants or antiplatelet agents (87).
Ticlopidine is a drug used to reduce the risk of thromboembolic attack in patients with a history of thrombotic lesions or high-risk patients. It is a platelet aggregation inhibitor that blocks the binding of platelets to fibrinogen by blocking ADP receptors on platelet membranes (79, 88). The combined use of ticlopidine, aspirin, and heparin effectively controls coagulation activation in patients with coronary dilation (77).
Aspirin inhibits the production of prostaglandin and prothrombotic thromboxane by the acetylation of COX-1 (78). It can also control the inflammatory conditions in CAE patients due to its anti-inflammatory effects (89). Dipyridamole, like aspirin, inhibits thromboxane formation and increases the extracellular concentration of adenosine by inhibiting adenosine reuptake. In addition, dipyridamole increases the concentration of platelet AMP by increasing adenylate cyclase activity and inhibiting the enzyme phosphodiesterase (90, 91). It has been suggested that the combination of aspirin with statins is more effective in controlling inflammation (92). In addition to the antithrombotic and lipid-lowering properties of aspirin and statins, respectively, they have anti-inflammatory properties; all two are essential factors in CAE development, and it is possible to simultaneously prescribe these two drugs to prevent internal vascular lesions.
Compared to aspirin, heparin can lead to platelet consumption and thrombocytopenia (93). Thrombocytopenia leads to the production of more active platelets and the formation of more coagulation clots in the arteries. As a result, aspirin seems to be better than heparin for CAE patients; however, both can control inflammation and reduce platelet adhesion and aggregation.
Clopidogrel is also an ADP-selective agent, and its anti-aggregation property is several times higher than that of ticlopidine (88, 94). Clopidogrel injection significantly increases streptokinase-induced thrombolysis, so the concomitant use of clopidogrel during streptokinase treatment may facilitate clot lysis (95). Antiplatelet therapies such as clopidogrel have shown clinical efficacy in many vascular diseases (96-98). Previous studies have reported that clopidogrel removal has been associated with proinflammatory effects such as increased levels of P-selectin and CRP (99, 100). As a result, it can be hypothesized that clopidogrel may also have anti-inflammatory effects in addition to preventing coagulation. However, the most effective dose and timing of its use are still debated, and the critical point is that increasing the dose of clopidogrel does not always prevent thrombotic events and individual platelet response to antiplatelet therapy. Individual platelet response depends on antiplatelet therapy, genetic, cellular, clinical, and environmental factors (78, 101, 102). For example, patients with older age and more coronary lesions show increased platelet activity and are more resistant to aspirin after treatment with clopidogrel (103). Also, in these individuals, after dual antiplatelet therapy via aspirin and clopidogrel, platelet reactivity increases, leading to cardiovascular events increment (104, 105). This variation in response to clopidogrel in patients with CAD may be associated with endothelial dysfunction. As a result, initiating the treatment of CAE patients with clopidogrel requires a detailed examination of the patient’s clinical characteristics; initiating the treatment without considering the patient and disease characteristics can worsen the condition.
CAE patients have more serum P-selectin, β-thromboglobulin, and platelet factor 4 than healthy individuals, indicating an increase in platelet activity and size (106). Larger platelets are more metabolically and enzymatically active than smaller ones and have faster aggregation (107). This increase in platelet size may be due to intravascular thrombosis in the ectatic segment, which reduces platelet consumption. Platelet depletion eventually leads to stress in megakaryocytes and the production of larger platelets (108, 109).
According to the above, failure to treat intravascular thrombosis can worsen patients’ clinical conditions; it also increases the platelet count. Platelets not only are the main axis of the coagulation system but also are involved in the inflammatory processes. Therefore, the continuous clot formation in CAE patients’ arteries leads to more platelet production, worsening inflammatory and coagulation conditions.
A study has shown that the injection of active platelets aggravates atherosclerotic lesions (110). Highly active platelets also cause more damage to endothelial function (111, 112). The vascular endothelium is one of the main factors to inhibit platelet aggregation and adhesion. The secretion of vasoactive mediators by active platelets may cause endothelial dysfunction (113), which may increase thrombotic events by inadequate production of nitric oxide and prostacyclin. They counteract platelet aggregation by increasing platelet activity (114). As a result, antiplatelet drugs can control the risk of increased platelet activity and vascular dysfunction.
Intravenous gamma globulin (IVGG) is a drug that can reduce the incidence of coronary aneurysms (115). It can also prevent abnormal coagulation by reducing fever and CRP. One study found that IVGG with aspirin was more effective than use it alone for coronary lesion. The action mechanism of this drug in improving vascular function is not yet known, but several studies have shown its effect on improving thrombosis and platelet aggregation (115). This drug can indeed have a positive effect on the coagulation control of CAE patients, but high doses of IVGG can increase platelet count (116). The dosage of this drug in CAE patients who have prone vessels to thrombosis is an important and controversial issue.
It is not clear why CAE increases the risk of thrombotic events. Endothelial dysfunction, slow blood flow, and the imbalance between coagulation and fibrinolysis can be the causes of this complication (117, 118). Inflammation and coagulation are two strongly related processes, and the produced factors in both mechanisms affect each other (119). For example, IL-6 and IL-3 increments can generate more reactive and larger platelets (120).
Inflammatory factors have a devastating effect on the coagulation system and endothelial cells function, which seems to cause thrombosis in CAE patients.
Given that platelets are the common center of inflammation and thrombosis (108), it can be argued that the platelet activity-related factors in CAE patients can have a prognostic role in the formation of thrombosis in these individuals. Evaluation of inflammatory parameters in these patients can also indicate the amount of coagulation activity; it also determines how to control the function of this system in CAE patients.
5. Angiotensin-Converting Enzyme and Beta-Blockers
Angiotensin-converting enzyme (ACE) and ACE2 are two involved enzymes in the pathogenesis of heart disease, and their functions are controversial during vascular disease. ACE converts angiotensin I to angiotensin II, and ACE2 cleaves angiotensin II (vasoconstrictor peptide) to produce angiotensin 1-7 (vasodilator) (33, 121). Angiotensin II is an effective peptide for vascular biology and inflammation that increases vascular permeability by stimulating the production of prostaglandins and vascular endothelial cell growth factor (VEGF) (122).
Monocytes/macrophages and dendritic cells increase in CAE patients and produce angiotensin II and express its receptor (123). Angiotensin II is a potent vasoconstrictor (especially in arteries) that can increase the expression of inflammatory cytokines and CRP genes in arteries and cardiac fibroblasts (124, 125). In contrast, angiotensin 1-7 has a wide range of anti-inflammatory and antioxidant effects. The balance between angiotensin II and angiotensin 1-7 is essential in vascular disease (126). Measurement of angiotensin II can indicate the CAE patients’ vascular conditions; in patients with an elevated plasma level of angiotensin II, the use of suppressive drugs can be fruitful.
Activation of angiotensin receptors leads to the production of vasodilators and nitric oxide. Cardiovascular therapy is based on methods that limit the production or binding of angiotensin II to its receptor. Valsartan is an angiotensin II receptor antagonist. It can reduce the fibrinogen level and improve endothelial function (127). However, studies have shown that valsartan can increase D-dimer, which is a risk factor for heart disease (128).
Telmisartan is also an angiotensin II receptor antagonist with a higher half-life and affinity than valsartan (129). Telmisartan can weaken the coagulation system by reducing endothelial and platelet markers and fibrinogen (130). The effects of both drugs on the coagulation and vascular systems are similar, but it seems that due to the longer half-life of telmisartan and the lack of increased D-dimer, it could be a better treatment option than valsartan (131).
The ACE inhibitors have beneficial effects in patients with heart problems, and their inhibition improves vascular endothelial function, possibly due to the lack of production of angiotensin II (132). Quinapril and ramipril are two ACE inhibitors that block the production of angiotensin II by ACE. These two drugs reduce the coagulation factors that are associated with the development of coagulation and clot formation in blood vessels; they also improve heart failure, heart regeneration, and recurrence of ischemic heart disease (133, 134).
One study found that lisinopril as an ACE inhibitor increased ACE2, which could benefit CAE patients. Studies have shown that the inactivation of ACE2 in mice causes severe cardiac dysfunction and increases the oxidative stress mediated by angiotensin II (135). Acquired or genetic deficiency of ACE2 leads to an increase in circulating angiotensin II or tissue angiotensin II.
Interestingly, ACE2 expression decreases atherosclerotic plaques, which can be associated with increased angiotensin II over time and worsening the patient’s clinical condition (136). Increased angiotensin II decreases ACE2 activity in the cardiac myocytes and ACE2 mRNA in cardiac fibroblasts (125). In conclusion, the inhibition of ACE2 and the adverse effects of its inhibition on cardiac function may be due to the persistence of angiotensin II and its lack of conversion. This suggests that angiotensin II downregulates ACE2. In contrast, an increase in ACE2 is associated with an increase in angiotensin II and angiotensin 1-7, which indicates that angiotensin II and angiotensin 1-7 can be considered the regulators of ACE2 in cardiovascular disease (137).
On the other hand, increased ACE2 expression is associated with increased angiotensin 1-7 and ventricular cardiac disorders (138). Angiotensin 1-7 is a vasodilator, and its increased level may be associated with ischemic cardiomyopathy. As a result, it can be hypothesized that an imbalance in ACE2 expression and activity is not conducive to cardiac function. Its inactivity is associated with the effects of angiotensin II, and its increased activity is associated with the adverse effects of angiotensin 1-7. Further and broader studies are suggested to reach definitive conclusions about the role of ACE2 in CAE patients.
Since ACE2 is expressed in the heart and its reduction is associated with a decrease in the heart’s pumping ability, the recombinant human enzyme ACE2 (rhACE2) is thought to be a new treatment for patients with vascular disorders (139). Evidence suggests that the treatment of coronary artery mice with losartan or olmesartan (angiotensin receptor antagonist) increases cardiac ACE2 mRNA and its activity (140). Although there is no convincing evidence for using this treatment in CAE patients, studies have shown that this enzyme improves systolic and diastolic right ventricular function (141). We hypothesize that using this enzyme during vascular occlusion surgeries or prescribing it to patients with vascular disorders can improve the blood circulation and coagulation system and play a cardioprotective role.
Treatment with lisinopril or losartan increases angiotensin 1-7 levels by increasing production or preventing its breakdown (142). The and DX600 are newly discovered ACE2 inhibitors. MLN4760 is more selective than DX600 for ACE2. Studies examining the effects of these drugs on heart disease have concluded that the inhibition of ACE2 worsens the disease (143). These drugs have not been studied in CAE patients.
Further studies on CAE patients are needed to reach a definitive conclusion about the effect of angiotensin-inhibiting drugs on vascular conditions. Genetic testing of ACE2 and ACE in CAE patients and measurement of angiotensin levels are considered essential measures which can be done to determine disease prognosis and the coagulation system. Therefore, achieving new and targeted drug therapies for CAE patients requires extensive clinical trials.
6. Surgical Procedures
When medical treatment is not enough to improve heart health, different surgeries are recommended for the patient. Percutaneous transluminal coronary angioplasty (PTCA) is usually performed in CAE patients. The doctor removes the coronary aneurysm in the PTCA procedure and improves vascular flow using a balloon-tipped catheter by pushing it into a blocked artery (144, 145). In many cases, after the artery has been opened, an expandable mesh stent is inserted into the artery to prevent future narrowing and re-occlusion of the arteries (146). In addition to PTCA, coronary artery bypass surgery is performed rarely. In this type of surgery, using a blood vessel that is removed from another part of the body, a blood vessel is placed in the form of a bypass at the site of the clogged artery (147).
Unlike coronary artery bypass surgery, stent placement is minimally invasive because it does not require incisions or injuries. This is often done under local or mild anesthesia and usually takes about an hour but may take longer if multiple stents are placed (148). Patients undergoing stent placement have much less pain and discomfort and a much shorter recovery time than patients undergoing coronary artery bypass surgery. Polytetrafluoroethylene-coated stents also appear to be more effective (149). However, angioplasty does not apply to everyone, and coronary artery bypass surgery may be a better option than angioplasty when the main artery that carries blood to the left of the heart is blocked, the heart muscle is weak, or multiple blood vessels are defective (150). For patients with diabetes or multiple clogged arteries, coronary artery bypass surgery may be a better option, too (151).
Although angioplasty is a less invasive procedure for opening blocked arteries than bypass surgery, it has some risks. The most common and significant risk of angioplasty is narrowing the artery (re-contraction). If the operation involves only angioplasty without stent placement, narrowing the artery occurs again in about 30% of the cases (152). Stents are designed to reduce the risk of recurrent artery occlusion. Uncoated metal stents reduce the chance of re-occlusion by up to 20% and drug-coated stents by less than 5% (153). Today, most angioplasty operations are performed with third-generation drug stents with a restenosis rate below 3%. Even after this operation, blood clots are possibly forming inside the stents. These clots can block the artery again and cause a heart attack. Therefore, anticoagulants or anti-inflammatory drugs are essential to reduce the risk of clot formation inside the stent. Also, measuring the patient’s angiotensin level before surgery can be a prognostic factor for the patient’s recovery process and vascular function; it can be prevented by using postoperative complications inhibitors.
7. Future Perspective
Inflammation is a pathological process that plays a vital role in developing multiple cardiovascular diseases. The association between inflammation and coagulation system and vascular endothelial function has also been established. According to the above, the incidence of CAE may improve due to a previous allergy or viral infection; they harm coagulation and Endothelial cell by stimulating the immune system and upsetting the balance between the production of inflammatory cytokines.
Therefore, anticoagulants and antithrombotic drugs can certainly be a way to prevent clotting events and improve endothelial function in CAE patients. In this study, we summarized some of the coagulation and inflammatory drugs to determine the effect of co-administration of these drugs on the recovery process acceleration of CAR patients. Also, identifying the pathogenesis of the disease can be effective in designing appropriate treatment methods to improve the patients’ response to treatment. In addition, the use of drugs along with surgery can be more effective in treating patients.