1. Background
Chronic pain can occur in almost any body part, and researchers have long investigated factors that cause chronic pain. Chronic pain is defined as pain lasting more than three months and is highly prevalent, occurring in 10 - 30% of the adult population, which is a considerable percentage (1). Chronic pain reduces motility, prevents physical activity, and leads to falls, depression, anxiety, sleep disorders, seclusion, loneliness, and disability (2).
The quality of sleep is influenced by diverse diseases, pain, and health status (3). Poor sleep quality involves disrupted sleep patterns, including a delay in falling asleep, experiencing problems in the stages of sleep, taking sleeping medications, and experiencing daytime drowsiness. Sleep is an organized and dynamic bio-cognitive process that constitutes a large portion of daily life (4), and its quality affects the quality of life as well as physical and mental health (5). Intrapsychic indicators associated with sleep quality, such as the degree of satisfaction with sleep and what is felt after waking up, are collectively defined as sleep quality (6, 7).
A variety of pharmacological and non-pharmacological methods are used to treat chronic pain. Medications are known as substances or combinations of them applied for treating, alleviating, preventing, or diagnosing physical problems and associated symptoms, as well as the materials that reconstruct, normalize, or alter the performance of human limbs (8). Gabapentin is an anticonvulsant agent prescribed for chronic and neuropathic pain. It is the medication of choice for neuropathic pain caused by diabetic neuropathy, post-herpetic neuralgia, and central pain (9). Transcranial direct current stimulation (tDCS) stimulates the central and peripheral nerves to raise intellectual ability and cognition. This is a unique and potentially effective way of treating depression, stress, anxiety, sleep difficulties, chronic pain, and substance misuse (10). Cranial electrical stimulation (CES) is a relatively straightforward procedure that utilizes a device with a small battery resembling a tDCS device. Although CES generates a different electrical current pulse than the tDCS device, it is effective (11). Numerous variables, namely current intensity, stimulation site, electrode size, stimulation duration, and current polarity, can significantly impact the outcome (12).
A current of only 1 A can provide sufficient electric stimulation for neural activity. Due to the resistance of the skin surface, to generate the required electric current, some clips are connected to the ears through which the direct current can generate an electrical resistance of 10000 - 40000 Ω in the skin under the electrodes and the body. This way, tDCS generates an electric current of 1 A that quickly activates the neurons (13). Tan et al., in two studies (14, 15), stated that tDCS is effective in alleviating pain intensity and is associated with fewer side effects. In a review study, Gilula and Kirsch (16) compared tDCS and pharmacotherapy and recommended that tDCS is more effective and has fewer side effects (16).
Acceptance and commitment therapy (ACT) is a third-wave behavioral therapy used to treat chronic pains (17). ACT can promote the sleep quality of people who have insomnia, thereby improving their psychological outcomes (18). ACT is based on personal values and focuses on accepting pain and suffering instead of avoiding and suppressing the factors that lead to discomfort. This approach aims to form more flexible reactions to life challenges, suffering, and pain and alters the functions of symptoms instead of eliminating them (19, 20). Using certain mechanisms, ACT helps people accept and regulate their unpleasant emotions instead of avoiding and suppressing them (21). According to Lin et al. (22), ACT alleviates the psychological distress and pain of women suffering from chronic lower back pain. It opens up new horizons in clinical interventions and can, therefore, serve as an influential intervention. Moreover, Zakiei et al. (23) concluded that therapists and health specialists could combine ACT with other therapies to improve sleep quality. Several other studies have also noted the effectiveness of ACT on sleep quality and duration (24-26).
2. Objectives
Chronic pain seems to affect the sleep quality of patients, which highlights the importance and necessity of this study. Accordingly, the present study aimed to investigate the role of ACT, tDCS, and pharmacotherapy on the sleep quality of women with chronic pain.
3. Methods
This randomized clinical trial study had a pretest-posttest follow-up design. The statistical population comprised all individuals with chronic pain visiting Pardis Pain Clinic, Tehran, Iran, in 2020. In the current study, the samples were selected through multistage random sampling out of the public and private centers related to chronic pain treatment in Tehran. A center (Pardis Pain Clinic) was randomly selected, and 60 women with chronic pain were selected from this center based on the inclusion criteria. The subjects were divided into four groups of 15 (ACT, pharmacotherapy, tDCS, and control) by the G*power statistical software with an effect size of 1.6, a test power of 0.9, and α = 0.05 (27). A random number table was used to randomly allocate the participants into three experimental groups and a control group. This way, one number between 1 - 60 (1 - 15 for the ACT group, 16-30 for the pharmacotherapy group, 31 - 45 for the tDCS group, and 46 - 60 for the control group) was considered for each participant by the researchers at pre-test. The inclusion criteria were having chronic pain, female gender, age of 35 - 60 years, high school education (in order to understand and answer the questionnaire), and consent to participation. The exclusion criteria were having severe psychiatric disorders diagnosed by clinical interviews based on the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5). After the random assignment of participants, the Pittsburgh sleep quality index (PSQI) was used as a pre-test in the control and test groups. The subjects in the experimental groups underwent ACT, pharmacotherapy, and tDCS. The control group did not receive any treatment. At the end of treatment sessions, all experimental and control group participants completed the PSQI as a post-test. Moreover, the four groups were followed up after 2 months. To avoid the bias of data transfer between groups, participants in groups 1 and 2 were treated on different days. For ethical considerations, the researchers received written consent from the participants for participation in the research. This article was extracted from a part of the Ph.D. dissertation of the first author. The present study followed a clinical trial design (IRCT20211026052879N1). Part of this Ph.D. dissertation has already been published (28).
3.1. Instruments
Pittsburgh sleep quality index (PSQI): The PSQI was developed by Buysse et al. in 1989 to evaluate sleep quality and patterns in one month. This 18-item questionnaire assesses seven components of subjective sleep quality, sleep duration, sleep disturbances, sleep latency, habitual sleep efficiency, daytime dysfunction, and the use of sleeping medication on a four-point Likert scale from 0 (not during the past month) to 3 (three or more times a week). Scores of 0, 1, 2, and 3 indicate highly desirable to undesirable states, respectively. The sum of the scores of the seven components ranges from 0 to 21, where 0 - 5 indicates desirable and 6 - 21 indicates undesirable sleep quality (29). The Persian version of the questionnaire was validated by Jahanshahi Hesari et al. (30).
3.2. Intervention Programs
3.2.1. Transcranial Direct Current Stimulation
The transcranial direct current stimulation (tDCS) involves increasing the delta waves (0.5 - 3 Hz) to raise endorphins and, therefore, alleviate pain and promote a sense of peacefulness. All the participants in the tDCS group received 20 min of delta wave at a 2 µA current for 10 weeks straight. Anode and cathode electrodes were placed on the frontal C3 and FP2 regions, respectively. Every 20-minute session started upon connecting the ear clips and automatically ended after a certain time elapsed. At the end of the 10th week, the participants were no longer allowed to continue treatment or receive any type of tDCS (31).
3.2.2. Pharmacotherapy
Pharmacotherapy involves taking 600 mg of gabapentin twice daily (300 mg in the morning and 300 mg at night) for three months (9).
3.2.3. Acceptance and Commitment Therapy
The acceptance and commitment therapy (ACT) was administered in eight one-hour weekly sessions. The content of the sessions was as follows:
Session 1: Introduction, the therapeutic agenda (establishing a therapeutic relationship, history taking, discussing the goals of research and treatment, reviewing previous therapies and their outcomes, changing the agenda from a tendency to control pain to more functional approaches, and giving assignments).
Session 2: Behavior change and mindfulness (discussing the potential values and choice, introducing the behavioral model, discussing behavior change, practicing mindfulness, feedback on mindfulness, giving assignments).
Session 3: Examining values (accepting painful personal events using metaphors, identifying and clarifying values, mindfulness, feedback, giving assignments).
Session 4: Clarifying values (discussing the barriers through metaphors, setting goals, introducing committed action, mindfulness and body scan, feedback, and giving assignments).
Session 5: Defusion (checking the assignments, coordinating movement and activity, defusing verbal threats, mindfulness, feedback, and giving assignments).
Session 6: Committed action (a review of the therapy, discussing committed action and the process of change, mindfulness, self-observation, and giving assignments).
Session 7: Satisfaction (a review of the assignments, defining primary pain as the primary damage, discussing satisfaction through allegories, commitment and barriers to satisfaction, mindful walking, and giving assignments).
Session 8: Conclusion [non-avoiding identification of values, preparing for (not preventing) relapse, adverse outcomes, and post-test using the questionnaire] (32).
3.3. Statistical Analysis
The mean and standard deviation were used in the present study to describe the data. The data were analyzed using repeated measures analysis of variance (ANOVA), Fisher’s exact test, Shapiro-Wilk test, Levene’s test, Box's M test, and Mauchly's Sphericity test for the pre-test. The SPSS software version 24.0 was used for statistical analysis.
4. Results
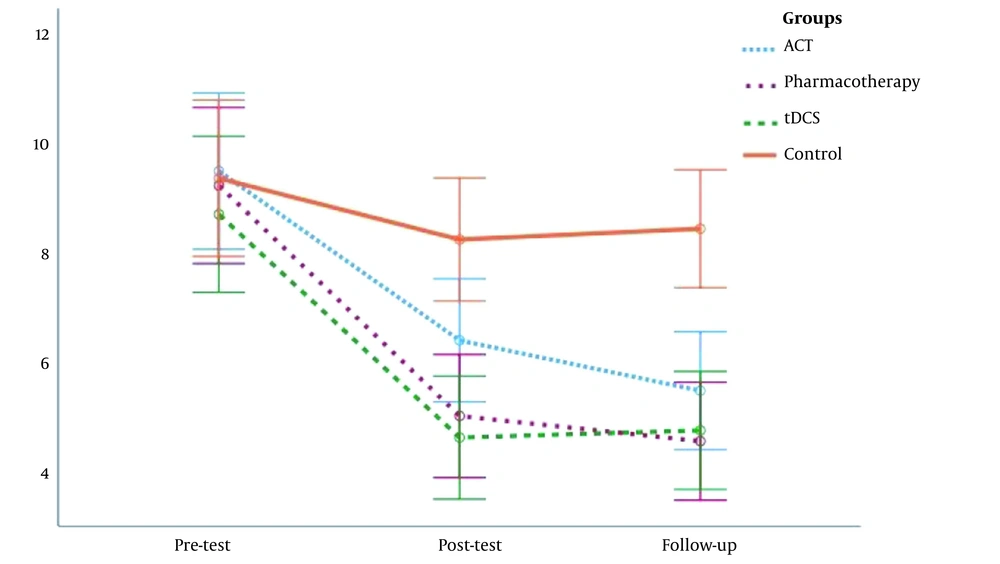
The participants aged 46.67 ± 8.39, 48.24 ± 7.54, 47.67 ± 7.42, and 47.39 ± 6.85 years in the ACT, pharmacotherapy, tDCS, and control groups, respectively. Table 1 presents the mean and standard deviation (SD) of sleep quality on the pre-test, post-test, and follow-up.
| Variables | Phases | ACT | Pharmacotherapy | tDCS | Control |
|---|---|---|---|---|---|
| Sleep quality | Pre-test | 9.47 ± 2.97 | 9.20 ± 2.24 | 8.67 ± 3.04 | 9.33 ± 2.87 |
| Post-test | 6.33 ± 2.13 | 4.93 ± 1.91 | 4.53 ± 1.77 | 8.20 ± 2.86 | |
| Follow-up | 5.40 ± 2.06 | 4.47 ± 1.55 | 4.69 ± 1.92 | 8.40 ± 2.75 |
The Shapiro-Wilk test for the dependent variable was insignificant at 0.05 in any group and at any stage of the study, indicating the normal distribution of sleep quality in all four groups and all three stages (pre-test, post-test, and follow-up). Levene’s test showed that the error variances of sleep quality scores did not significantly differ between the four groups and the three stages. Therefore, the assumption of the homogeneity of error variances in the sleep quality was confirmed. One-way ANOVA was run to assess the independence of sleep quality from group membership on the pre-test in the experimental and control groups [F (3, 56) = 0.24, P > 0.05]. Before the intervention, the test and control groups were not significantly different in terms of sleep quality, demonstrating the independence of the variable from group membership on the pre-test.
The group × time effect on sleep quality [F (6, 110) = 5.63, P = 0.001, η2 = 0.235, Wilks' lambda = 0.585) was significant. The assumption of sphericity or the equality of error variance matrices was tested by Mauchly’s test and was confirmed with a non-significant chi-squared value for the dependent variable [χ2 (2) = 4.53, W = 0.921, P > 0.05]. Table 2 presents the mixed-design analysis to explain the effects of independent variables on sleep quality. The group × time effect on sleep quality was significant [F (6, 112) = 6.51, P = 0.001, η2 = 0.259].
| Variable | SS | SSE | df | F | P | η2 |
|---|---|---|---|---|---|---|
| Sleep quality | 60.48 | 79.02 | 6, 112 | 6.51 | 0.001 | 0.259 |
Pairwise comparisons were performed to compare the effects of independent variables with each another and with the control group (Table 3). The results revealed that ACT [F (2, 56) = 7.76, P = 0.001], pharmacotherapy [F (2, 56) = 17.97, P = 0.001], and tDCS [F (2, 56) = 9.37, P = 0.001] promoted sleep quality compared to the control group. The effects of ACT, pharmacotherapy and tDCS on the sleep quality of patients with chronic pain did not significantly differ.
| Variable | Groups | SS | SSE | df | F | P | η2 |
|---|---|---|---|---|---|---|---|
| Sleep quality | ACT-Pharmacotherapy | 4.87 | 89.07 | 2, 56 | 1.53 | 0.225 | 0.052 |
| ACT-tDCS | 5.36 | 103.32 | 2, 56 | 1.15 | 0.324 | 0.039 | |
| ACT-Control | 37.76 | 136.22 | 2, 56 | 7.76 | 0.001 | 0.217 | |
| Pharmacotherapy-tDCS | 2.29 | 90.38 | 2, 56 | 0.81 | 0.496 | 0.025 | |
| Pharmacotherapy-Control | 61.79 | 96.22 | 2, 56 | 17.97 | 0.001 | 0.391 | |
| tDCS-Control | 46.02 | 137.51 | 2, 56 | 9.37 | 0.001 | 0.251 |
Figure 1 shows a diagram of sleep quality in the pre-test, post-test, and follow-up stages in four research groups. The mean sleep quality scores of the three test groups diminished on post-test and follow-up compared to pre-test, while no difference was observed in the control group.
5. Discussion
The present study aimed to investigate the effectiveness of ACT, tDCS, and pharmacotherapy on the sleep quality of women with chronic pain. The results showed that ACT, pharmacotherapy, and tDCS significantly improved the sleep quality of patients with chronic pain compared to the control group. These results are consistent with the findings of Salari et al. (18) and Zhou et al. (33). Moreover, Salari et al. (18) reported that ACT could significantly improve sleep quality in patients with primary insomnia. Zhou et al. (33) suggested that tDCS stimulation not only enhanced the symptoms of depression and anxiety but also had a positive effect on the sleep quality of patients with major depressive disorder. The impacts of ACT, pharmacotherapy, and tDCS on the sleep quality of patients with chronic pain were not significantly different. ACT encourages people to identify their values, actions, and barriers, along with setting goals, being committed to performing those actions to reach their goals, and moving in line with their values despite barriers. By realizing one’s goals, the ensuing happiness promotes people’s satisfaction with life and prevents them from falling into the loop of negative thoughts and feelings that exacerbate their problems (20). Emotion regulation helps people effectively cope with and respond to stressful situations. Those who participate in ACT courses are less impacted by stressful situations due to the formation of values and meaning in their lives and can regulate and manage their emotions more effectively (34).
The acceptance achieved through mindfulness and paying attention to internal experiences in ACT does not equate with the desire for hurtful experiences and emotions, merely tolerating them, or showing resistance to them; instead, it means the tendency to experience unpleasant events (ie, internal events that occur when acting in line with values). Acceptance (the opposite of experiential avoidance) is a fundamental element of psychological flexibility (20). Mindfulness aims to promote the experience of emotions and cognitions as internal events that serve as a response to external and internal stimulants. ACT assumes that people’s language and cognition disrupt and often intensify emotion-ridden experiences. This becomes problematic when people try to circumvent language-dependent processes. Therefore, acceptance happens following cognitive defusion and viewing oneself as a context (21) that regulates unpleasant emotions (34). Mindfulness enables people to perceive and process events around them to make rational decisions and properly react to difficult situations. It allows people to externally observe their emotions and cognitions separate from the inside or the outside world (35). Clients are encouraged through acceptance to welcome their feelings and thoughts without "experiential avoidance," resistance, or suppression (36). Overall, ACT helps people get in touch with a transcendental sense of self and view the self as a context that is constantly observing and experiencing, separate from thoughts, feelings, bodily sensations, and memories. Avoidance in people with chronic pain may diminish their sleep quality. In the ACT, acceptance provides an alternative to experiential avoidance. Acceptance involves an active and conscious tendency to experience uncontrollable events without trying to change them, especially when this effort leads to more psychological harm (21).
Pharmacotherapy affects the chemical balance in the brain to alleviate or eliminate the symptoms of disorders. Researchers believe that signs, symptoms, and mental experiments associated with mental disorders indicate central nervous system (brain) dysfunction, which is the outcome of electrochemical imbalance in the brain. Any cephalic activity results from chemical molecules that influence, stimulate, or inhibit neurons as neurotransmitters. In pharmacotherapy, medications serve as, enhance, or inhibit chemical molecules or natural neurotransmitters. This way, pharmacotherapy mitigates or eliminates the symptoms of mental disorders (36).
The tDCS improves serotonin balance by modulating particular alpha brain waves. The tDCS modulates blood flow between the left and right hemispheres of the brain, consequently regulating the limbic system, thalamus, and basal ganglia, all of which regulate serotonin and contribute to chronic pain alleviation. Furthermore, tDCS affects the brain, influences hormone and neurotransmitter levels in the blood, and enhances monoamine oxidase activity and gamma-aminobutyric acid blood concentrations. This approach enables the delivery of a direct current to the brain via the brainstem, limbic system, network activator systems, or hypothalamus (37). As a result, neurotransmitter production is influenced, as is the activity of the default network mode, which is almost certainly the default network, and a large-scale neural network composed of sections with closed activities insulated from other neural networks.
This study was conducted on people with chronic pain referred to Pardis Pain Clinic, Tehran. Therefore, our results could be generalized to other groups with chronic pain or patients with psychiatric disorders with caution.
5.1. Conclusions
The findings of the current research demonstrated that improving signs and symptoms through pharmacotherapy and tDCS can reinforce the effects of other therapies, including psychotherapy. Evaluation and explanation of the impacts of ACT, tDCS, and pharmacotherapy on psychological characteristics, such as the sleep quality of women with chronic pain, are among the most important strengths of this study. Still, as medications cannot definitively and completely treat mental disorders, physicians and health specialists are recommended to combine ACT, pharmacotherapy, and tDCS to treat chronic pain, promote mental empowerment, accelerate recovery, and help patients manage their treatment. Evaluation and comparison of the impact of three therapeutic interventions, ACT, tDCS, and pharmacotherapy, on the sleep quality of women with chronic pain are the most important innovations of the present study, which have not been addressed in previous investigations. Consequently, the current research provides an opportunity to investigate the effectiveness of these treatments and also design and provide efficient services and interventions for patients with chronic pain.