1. Background
Acute kidney injury (AKI) is usually defined as a syndrome in which the renal function deteriorates dramatically during a period of hours to days, and is accompanied by an increase in the nitrogenous waste products in the blood. Other findings include a decrease in urine volume, as well as an accumulation of metabolic acids and other compounds such as phosphate or potassium (1). Many kidney functions such as osmosis regulation, pH regulation, waste products disposal, and hormonal catabolism may be disrupted due to AKI, and other functions such as hemostatic capabilities and blood pressure regulation by the kidneys may also be affected. Considering the wide range of events and outcomes that may follow AKI, proposing an exact definition encompassing all this complexity may prove challenging. Furthermore, the actual damage to the kidney can happen prior to, during, or after the loss of function (2).
Acute kidney injury has been substituted for acute renal failure (ARF) in order to highlight the so-called injuries that take place prior to the decrease in function. These injuries are often detectable by laboratory tests and can be treated if diagnosed early (3). Taking into account the lab results, AKI is defined as a 0.3 mg/dL increase in serum creatinine from the base level in the first 24 hours, or a total increase of more than 50% in the first 48 hours (4). Studies have shown that a twofold increase in serum creatinine is correlated with 2 - 5 times increase in mortality. Even after the improvement of renal function, the patients are still at the risk of chronic kidney disease and early mortality (5).
Despite recent advances in diagnosis and treatment, AKI remains a challenge for the healthcare workers worldwide (6). Several epidemiologic studies have been carried out in different regions of the world in order to evaluate its prevalence. A study conducted in Lithuania documented a prevalence of approximately 4 - 6% for AKI, where roughly 10% of the patients eventually required dialysis (7). Another study reported a prevalence of 5% for AKI. The situation worsens significantly when the patients are admitted to the intensive care unit (ICU). Two large cohort studies have recorded a prevalence of about 36% for AKI in ICU admitted patients, which seems to be increasing (8, 9).
Defined as a dysregulated reaction to severe and disseminated infection, sepsis is regarded as a main cause of death in developing countries and is the third cause of death from infection only after pulmonary infections and AIDS. Sepsis is believed to be the most frequent cause of mortality in ICU patients, a risk factor for AKI, and the first cause in term of frequency of occurrence. Approximately 50% of the patients with AKI also have sepsis, and the occurrence of AKI in septic patients has been argued to increase the likelihood of mortality by 30 - 50% (10-12).
Most studies have investigated the infants and young patients, and very few studies have explored the occurrence of AKI caused by sepsis in adult patients. To address this problem, Fitzgerald et al. conducted a study and showed that the identification of some risk factors, such as the age of the pediatric patient, the nature of the bacterial infection, the type of antibiotic used, and the drop in blood pressure may have facilitated the management of AKI in high-risk patients (13). The study by Cai et al. found that the adoption of appropriate treatment strategies to prevent the progression of AKI in neonatal infants with septicemia may have positively contributed to their proper management (14).
2. Objectives
Taking into account the significance of the given issue and the lack of national prior data, the current study aimed to investigate the correlation of different factors with occurrence of AKI in ICU admitted patients diagnosed with sepsis.
3. Methods
3.1. Patient Selection
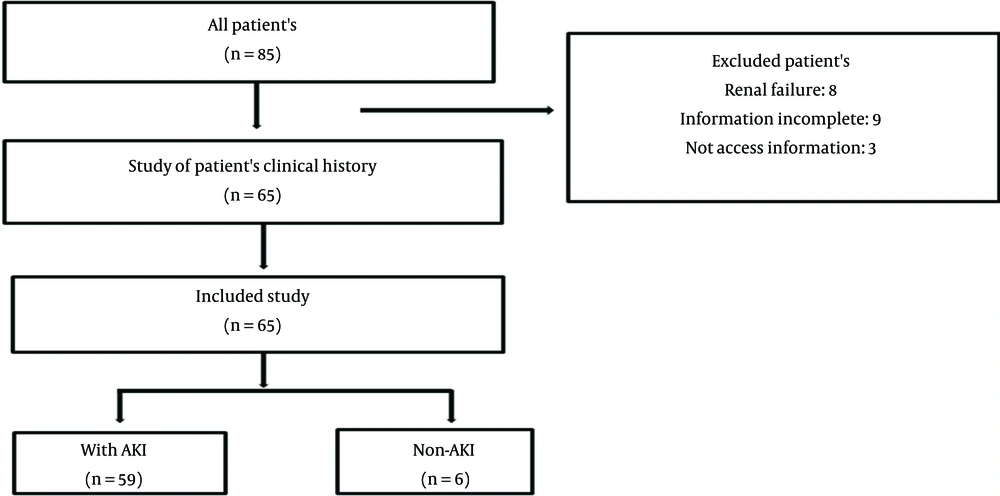
A total of 65 patients (22 female) were included in this retrospective study. Patients' files were randomly selected based on availability (Figure 1), and their data were accessed and collected via checklists of patients admitted to the ICU during a period of approximately two years. Patient outcome was assessed in a 28-day period beginning with the admission to the ICU.
3.2. Inclusion and Exclusion Criteria
The inclusion criteria included adult patients of both sexes diagnosed with sepsis and admitted to the ICU. The exclusion criteria were the age under 18, absence of sepsis, presence of AKI prior to the onset of sepsis, sepsis accompanied by rhabdomyolysis or ischemia, and septic shock.
3.3. Defined Sepsis and AKI Criteria
Sepsis was defined as a dysregulated response to infection resulting in a life-threatening dysfunction in a vital organ based on the surviving sepsis campaign guideline 2016 (12), and was diagnosed based on clinical and laboratory findings.
AKI was defined as a 0.3 mg/dL increase in serum creatinine from the base level in the first 24 hours, or a total increase of more than 50% in the first 48 hours. The severity of AKI was assessed using the RIFLE scale in which the severity is classified as risk (class R), injury (class I), and failure (class F), and the two outcomes followed by reaching a class F disease are Loss and End-stage kidney disease (ESRD) (4, 15).
QSOFA, a set of criteria used for assessing end organ damage severity and probability of mortality following sepsis, was calculated so that the respiratory rate of more than 22, A Glasgow Coma Scale (GCS) of less than 15, and systolic blood pressure of less than 100 were given points; and a total score of more than 2 was corelated with a 10% chance of mortality, and an overall mortality 2 to 25 times that of patients with a QSOFA score of less than 2 (16).
3.4. Information Collection
Patient comorbidities, sepsis origin, antibiotic treatment, serum creatinine level, C Reactive Protein (CRP) levels, need for hemodialysis during the study period, and eventual patient outcome was also recorded. Moreover, 14-day and 28-day mortalities were also recorded. Finally, the correlations between different factors and the occurrence of AKI were analyzed. The collection of data about the patients was performed using a checklist.
3.5. Ethical Section
Our study protocol was reviewed and approved by the ethics committee of Tehran University of medical Sciences under approval number IR.TUMS.IKHC.REC.1398.134. Furthermore, written informed consent was obtained from participants or their legal guardians before their participation in the study.
3.6. Sample Size and Statistical Analysis
According to the study, the mortality rate in the two-year follow-up of the patients with acute renal failure with sepsis was calculated as 34% (P = 0.34) using the following formula:
Considering the relative error of 30%, the sample size was determined. According to the formula, the sample size was equal to 65 individuals.
The data were analyzed by SPSS software version 22. Then the parametric or non-parametric statistical tests were used based on the normality or non-normality of the distribution of the variables.
4. Results
4.1. Demographical Information
As shown in the Table 1, the average age of patients with AKI was higher than that of non-AKI patients. In addition, the levels of CRP, Cr, and SOFA at ICU admission were higher in patients with AKI compared to non-AKI patients. However, no significant difference was observed between them (P-value > 0.05) (Table 1).
| Assessed Factors | Non-AKI, Mean ± SD | AKI, Mean ± SD | P-Value a |
|---|---|---|---|
| Age | 45.0±20.45 | 63.28±18.48 | 0.08 |
| CRP at ICU admission | 55.83±26.33 | 71.11±34.90 | 0.23 |
| SOFA at ICU admission | 3.43±1.0 | 9.15±6.0 | 0.007 |
| Cr at ICU admission | 0.85±0.22 | 0.95±0.21 | 0.60 |
| Baseline Cr | 0.86±0.23 | 0.92±0.28 | 0.58 |
Association Between Quantitative Variable and AKI Occurrence in Sepsis Patients
4.2. AKI Status and Outcome
Out of a total of 65 patients included in the study, 59 were diagnosed with AKI during the study period. Of those with AKI, 27 ones were class R, 16 ones were class I, and 16 ones were class F. As for the outcome of AKI, 35 patients recovered during the four-week period, and six patients needed at least one session of dialysis. Moreover, the mortality rate was 13.8% (9 patients) in the 14-day period and 43.1% (28 patients) in the 28-day period.
4.3. Evaluation of Underlying Disease in Sepsis Patients
According to Table 2, the prevalence of underlying diseases, including cardiovascular disease, diabetes mellitus, pulmonary disease, malignancy, and hypothyroidism was higher in patients with AKI compared to non-AKI patients. However, no significant difference was observed between them (P-value > 0.05).
| Underlying Disease | Non-AKI (n = 6) | AKI (n = 59) | P-Value* |
|---|---|---|---|
| Cardiovascular disease | 1 | 33 | 0.09 |
| Diabetes mellitus | 2 | 12 | 0.60 |
| Pulmonary disease | 0 | 6 | 1.00 |
| Malignancy | 0 | 10 | 0.58 |
| Hypothyroidism | 0 | 5 | 1.00 |
Association Between Underlying Disease and AKI Occurrence in Sepsis Patients
4.4. Evaluation Of Antibiotics Consumption in Sepsis Patients
Table 3 shows a list of antibiotics prescribed to patients with sepsis. According to this table, the consumption of antibiotics was higher in patients with AKI compared to non-AKI patients. A significant relationship was observed between the two groups in terms of Vancomycin antibiotic application (P-value: 0.006).
| Antibiotics Consumption | Non-AKI (n = 6) | AKI (n = 59) | P-Value a |
|---|---|---|---|
| Vancomycin | 2 | 47 | 0.006 |
| Piperacillin-Tazobactam | 2 | 21 | 0.87 |
| Carbapenem | 5 | 44 | 0.73 |
| Polymyxin | 2 | 14 | 0.45 |
| Levofloxacin | 1 | 0 | 0.54 |
| Aminoglycoside | 1 | 17 | 0.50 |
Association Between Antibiotics Consumption and AKI Occurrence in Sepsis Patients
4.5. Evaluation of Source of Infection in Sepsis Patients
Table 4 lists the sources of infection in patients with sepsis. According to this table, the highest source of infection in patients with AKI was related to respiratory infection, while the lowest one was related to Meningitis. However, no significant relationship was observed between them (P-value > 0.05).
| Infection Source | Non-AKI (n = 6) | AKI (n = 59) | P-Value a |
|---|---|---|---|
| Skin and soft tissue | 0 | 3 | 0.57 |
| Respiratory infection | 4 | 31 | 0.54 |
| Urinary infection | 0 | 16 | 0.14 |
| Bacteriemia | 5 | 28 | 0.10 |
| Meningitis | 0 | 2 | 0.64 |
Association Between Source of Infection and AKI Occurrence in Sepsis Patients
4.6. Evaluation of Logistic Regression for risk Factors and AKI
Our study results indicated that the application of vancomycin to treat patients increased the incidence of AKI risk in patients by 2.24 times, and a statistically significant relationship was detected between them (Table 5).
| Variable | Group | Odd ratio | CI 95% | P-Value |
|---|---|---|---|---|
| Vancomycin | No | Reference | ||
| Yes | 2.24 | 1.5 - 58.5 | 0.016 a |
Evaluation of AKT with Vancomycin Using Logistic Regression
5. Discussion
AKI following sepsis is considered a relatively frequent complication seen in ICU admitted patients, which can cause considerable morbidity and mortality. Sepsis-related AKI has been found to result in a higher increase in mortality and morbidity when compared with AKI caused by other factors (17). Furthermore, kidney function markers (e.g., creatinine level) are usually unable to provide early diagnosis of AKI.
In the present study, the occurrence and clinical aspects of AKI in septic patients admitted to the ICU were investigated. Due to the COVID-19 pandemic, a large portion of ICU beds were allocated for the treatment of COIVD patients and, therefore, ultimately 65 patients were included in the study.
Out of 65 patients included in the study, 59 were diagnosed with AKI. The AKI classification was performed using the RIFLE classification. Since most studies adopt RIFLE to assess the severity of AKI, the original scores are sometimes modified, which usually results in the production of different outcomes. According to the evidence, the RFILE scoring system is comparable to the most favorable system for producing a correct and accurate classification of AKI (5), as opposed to other methods such as acute kidney injury network (AKIN) score (18). Overall, the difference in population, ICU admission criteria, and AKI severity classification can all lead to differences in the final reports results.
Presently, the researchers are looking for clinical or laboratory markers able to facilitate early diagnosis and assessment of the prognosis of AKI because markers with high sensitivity and specificity can help develop new preventive and early treatment plans, which, in turn, can have a significant impact on patient outcomes. To date, they have identified a number of markers which may prove useful in the early detection and prognosis of AKI (8, 19).
Considering the source of sepsis, respiratory infection was found to be the most significant factor in our study, which was consistent with the findings from other similar studies (7). As for the correlating factors, the QSOFA score and Vancomycin administration were the only factors significantly correlated with the occurrence of AKI. In a similar study, Zhi et al. reported that patients with AKI related to sepsis showed a more severe disease, and presented with more frequent anomalies in vital signs and laboratory data (e.g., AST, ALT, creatinine, etc.). Furthermore, these patients had higher SOFA scores, which was in line with our findings (17). A correlation between age and AKI has also been reported in the literature (20), but such a correlation was not observed in our study.
As discussed above, sepsis-related AKI is correlated with poorer clinical outcomes. These include a higher risk of mortality (odds ratio of 1.48) and longer hospital stay (37 as opposed to 21) (20). Of the patients with sepsis-related AKI, those needing renal replacement therapy were found to be highly correlated with higher mortality (11), while those recovered from AKI had a significantly improved survival (21). Sepsis-related AKI was also associated with wider application of healthcare resources and higher medical costs, and the patients had longer hospital stays (7). Fourteen-day and 280-day mortality rates in our study were 13.8% and 43.1%, respectively, which were in agreement with the results from a study by Zhao et al. reporting that the 28-day mortality rate in sepsis-related AKI was 42.9% and was significantly higher than mortality rate in septic patients without AKI (22).
Vancomycin administration was also found to be significantly correlated with AKI. Vancomycin is a tri-cyclic glycopeptide antibiotic discovered in 1958. In this regard, a study provided limited evidence suggesting that the application of Vancomycin may have been associated with higher probability of AKI in such a way that more than half of the patients developed AKI likely due to receiving Vancomycin. Even though significant, this is still considerably less than known nephrotoxic antibiotics such as Aminoglycosides and Non-liposomal Amphotericin B (23). Interestingly, no correlation was observed between Aminoglycosides application and AKI in our study.
The present study attempted to further investigate the correlation between different factors and AKI in septic patients; however, it faced few limitations. First, it was a single-center study and was affected by the complications regarding the COVID-19 pandemic. Second, it was retrospective by its very nature. Therefore, it was recommended that further studies should be carried out in order to facilitate identifying and understanding the contributing factors and mechanisms involved in sepsis-related AKI.
5.1. Conclusions
Our study investigated the factors correlated with sepsis-related AKI in ICU-admitted patients with a 28-day follow-up period. The results suggested the correlation of higher QSOFA scores and Vancomycin administration with AKI. Our study results may have facilitated managing these patients in the future since septic patients should be managed and provided with care based on their QSOFA scores as well as on the administration of Vancomycin and other nephrotoxic.
5.2. Limitations
In this study, the number of examined patients was small; therefore, it was recommended that further studies with larger sample sizes should be conducted. In this study, the effect of drugs on the occurrence of AKI was not investigated. Moreover, the relationship between the type of sepsis-causing bacteria and the occurrence of AKI was not investigated.