1. Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in Wuhan, China 2 years ago. According to the World Health Organization (WHO), over 200 million individuals have been affected by the virus, and nearly 5 million patients have died worldwide. Finding a treatment to prevent patients from getting chronic diseases, such as pneumonia, acute respiratory distress syndrome (ARDS), and multi-organ dysfunction, is one of the most critical issues to consider (1). At the beginning of the epidemic, different treatments, including interferon-gamma, antibiotics, and anti-viral drugs, were applied to decrease the viral load in patients. Medications that have been used recently, such as remdesivir, favipiravir, and nafamostat, showed average results in infection control (2); however, none had a significant impact on preventing the disease. Another treatment that has been approved by the WHO is using plasma from recovered coronavirus disease 2019 (COVID-19) patients.
Convalescent blood products (CBP) obtained from patients surviving an infection have been mentioned as a suitable source of neutralizing antibodies (NAbs) against pathogens (3, 4). The CBPs could help neutralize and eradicate pathogens from patient blood. To date, various types of CBPs, such as convalescent plasma (CP), immunoglobulin (Ig), and antibodies, have been used (5, 6). The CP has attracted the greatest attention, especially in treating and controlling large-scale epidemics (7). The CP was first introduced as a potential treatment for viruses in a study on the Spanish flu, the results of which showed a significant reduction in mortality (8). Since then, CP has been widely used to control other viral infections, such as cytomegaloviruses, influenza, and large epidemics caused by coronaviruses, including Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS) (9-11).
Considering the clinical similarities among SARS, MERS, and COVID-19, utilizing CP might be an excellent way to control SARS-CoV-2. In a study in China, CP of clinically recovered COVID-19 patients was used to treat patients with viremia. The results indicated that this method could treat infected patients and lead to a faster recovery (12). On the other hand, CP received from donors contains NAbs, inflammatory cytokines, and chelating factors. Due to the critical role of NAbs in the clearance of viruses, CP obtained from recovered patients can provide these infection-inhibitory antibodies. Additionally, other antibodies, proteins, and inflammatory cytokines can effectively modulate the immune system (13). Therefore, using CP as a treatment to reduce and control infection in COVID-19 patients is suggested.
Erythrocyte sedimentation rate (ESR) (14), lactate dehydrogenase (LDH) (15), C-reactive protein (CRP) (16), D-dimer (17), platelet (PLT), and lymphocyte count (18) are clinical biomarkers for the diagnosis of COVID-19 infection. Considering the role of these laboratory indicators in COVID-19 and the difference in their levels in different categories of disease severity (asymptomatic to severe respiratory failure) (19), the use of CP can be effective in these clinical factors. Therefore, assessing the effect of CP on these laboratory data can be helpful in determining whether this method can modify disease severity.
Since the CP of SARS patients carries antibodies against SARS-CoV-2 and might suppress viremia, it has been suggested that CP might be an effective treatment option for patients with severe SARS-CoV-2 infection. Patients who have recovered from this infection with a high NAb titer (NAT) provide a valuable source of CP, and the absence of NAbs against this novel virus in the general population provides a unique opportunity to study its effect on the treatment of severe cases. Regarding the effectiveness of CP therapy, similar to other treatments, and its beneficial effects on previous viral infections and epidemics, we decided to explore the feasibility of this treatment by evaluating the laboratory data of COVID-19 patients.
2. Objectives
Considering that individuals who have recovered from COVID-19 have NAbs in their serum, and NAbs have a therapeutic effect, the aim and novelty of this study are to investigate the effects of CP on inflammatory factors as a biomarker to check the health status of patients.
3. Methods
3.1. Patients and Ethical Considerations
This study was conducted on 125 patients with COVID-19 admitted to the Intensive Care Unit (ICU) of Ali-Asghar Hospital in Shiraz, Iran, from April 10 to November 20, 2020. The patients were divided into a receiving-plasma group (n = 100) and a not-receiving plasma group (n = 25).
3.2. Inclusion Criteria
The patients were within the age range of 30 - 70 years in this study. The COVID-19 infection was confirmed by polymerase chain reaction (PCR), and pneumonia was confirmed by a chest X-ray. Informed consent forms were signed by the patients.
Plasma donors were the patients who had fully recovered from COVID-19 and were discharged from the hospital more than two weeks ago. The inclusion criteria for the donors were negative COVID-19 PCR test results (twice with a 24-hour interval), no persistent COVID-19 symptoms, and an age range of 30 - 70 years.
3.3. Exclusion Criteria
The patients were excluded if they had a history of hypersensitivity to blood products, such as plasma and its associated compounds (sodium citrate), or were under critical conditions, such as severe organ dysfunction that is unsuitable for plasma injection. The donors had to meet blood transfusion criteria according to the Blood Transfusion Organization guidelines, including the absence of heart diseases, diabetes, or viral infections, such as human immunodeficiency virus and hepatitis B virus, which were screened by the organization before receiving blood. Otherwise, they were excluded from the study. The information required for the study was collected with the help of two experts to eliminate data extraction errors. This study was conducted based on the recommendations of ethical guidelines. The research protocol was also authorized by Shiraz University of Medical Sciences (IR.SUMS.REC.1399.020).
3.4. Convalescent Plasma Transfusion
The CP collection was performed according to the routine plasma collection procedures via plasmapheresis. The plasma products were prepared as fresh-frozen plasma. The COVID-19 CP was collected and processed at Fars Blood Transfusion Organization. A receptor-binding domain-specific immunoglobulin G antibody titer was measured for the CP products and was reported as less than 1: 160, 1: 160, 1: 320, and 1: 640 or greater than 1: 640. The dosage of COVID-19 CP transfusion was almost 4 - 13 mL/kg of the recipient’s body weight. It is worth mentioning that the ABO type of the transfused CP was compatible with the patient’s ABO type. In addition, CP was crossmatched with the patient’s red blood cells to ensure compatibility. The CP was transfused at approximately 100 mL per hour with close monitoring. Adjustments in the infusion rates were allowed based on the risk of volume overload and tolerance according to the treating physicians’ discretion. No premedication was given before the CP transfusion.
3.5. Hematological and Biochemical Assays
The COVID-19 patients’ laboratory data, including LDH (Biorex, Iran, cat No. BXC0242A), CRP (Biorex, Iran, cat No. LXCRP150), ESR (Automated Modified Westergren method), PLT count, lymphocyte count, and D-dimer (VIDAS, bioMérieux, France) were retrieved from their hospital records on the day of CP transfusion and 4 and 7 days later.
3.6. Statistical Analysis
The data were statistically analyzed using SPSS software (version 26.0). The symptoms and drugs were compared using the chi-square test. Vital signs on admission were reported as mean ± standard deviation (SD). Additionally, para-clinical findings were reported as mean ± SD and compared via the independent sample t-test. A P-value less than 0.05 was considered statistically significant.
4. Results
4.1. General Characteristics of Patients
Table 1 shows the basic information about the patients (receiving plasma) and controls (not receiving plasma). Among the patients, 79 (63.7%) and 44 (35.4%) subjects were male and female, respectively. In the control group, 19 and 6 patients were male and female, respectively. In addition, the mean age of the male patients was 62.6 and 63 years in the CP-treated and control groups, respectively. The mean age of the female patients was also 56.8 and 67.5 years in the CP-treated and control groups, respectively. Considering the symptoms, shortness of breath was significantly common, and cough and fatigue were observed to a large extent among the patients. Remdesivir, Kaletra, favipiravir, and antibiotics were the medications that were given to the patients during hospitalization. The mortality rate was higher in the control patients who did not receive plasma (84%) than in the patients receiving plasma.
| Variables | CP-treated Patients | Control Patients |
|---|---|---|
| Age (y) | ||
| < 50 | 20 (20.4) | 6 (24) |
| ≥ 50 | 71 (72.4) | 19 (76) |
| Gender (male/female) | 62/36 (63.2) | 17/8 (68) |
| Outcome (expired) | 52/36 (53) | 21/4 (84) |
| Vital signs | ||
| Oxygen saturation (%) | 77.27 ± 12.8 | 75.75 ± 14.5 |
| Systolic blood pressure (mmHg) | 127.07 ± 20.7 | 135.5 ± 19.9 |
| Diastolic blood pressure (mmHg) | 76.66 (12.8) | 83.7 (18.5) |
| Temperature (°C) | 36.8 (0.84) | 36.8 (0.75) |
| Pulse rate (beats/min) | 97.42 (16.8) | 102.25 (22.8) |
| Respiratory rate (beats/min) | 21.58 (9.5) | 23.4 (14.3) |
| Symptoms | ||
| Cough | 46/53 (46.5) | 13/12 (52) |
| Weakness | 46/53 (46.5) | 14/11 (56) |
| Fever | 37/62 (37.4) | 7/18 (28) |
| Dyspnea | 83/16 (83.8) | 25/0 (100) |
| Loss of appetite | 17/82 (17.2) | 2/23 (8) |
| Diarrhea | 7/92 (7.1) | 3/22 (12) |
| Nausea/vomiting | 10/87 (10.1) | 7/18 (28) |
| Body pain | 13/86 (13.1) | 12/13 (48) |
| Fatigue | 4/95 (4) | 0/25 (0) |
| Headache | 9/90 (9.1) | 1/24 (4) |
| Treatment | ||
| Remdesivir | 59/40 (59.6) | 16/9 (64) |
| Favipiravir | 11/88 (11.1) | 3/22 (12) |
| Antibiotic | 16/83 (16.2) | 3/22 (12) |
| Kaletra | 45/54 (45.5) | 11/14 (44) |
| Nutritional supplements (vitamins C, B, and D and zinc) | 64/36(64) | 20/5(80) |
Abbreviation: CP, convalescent plasma.
a Values are expressed as No. (%) or mean ± standard deviation.
4.2. Changes in Clinical and Laboratory Parameters Before and After CP Therapy
Table 2 shows the changes in the initial parameters of the patients who received plasma on the day of CP administration and 4 and 7 days later and the patients of the control group on 4 different days during hospitalization. The findings revealed a significant difference in the amount of D-dimer (P = 0.036). Significant changes were also observed in oxygen saturation (SpO2) (P = 0.00) lymphocyte count (P = 0.00), PLT count (P = 0.005), and ESR (P = 0.007) on the fourth day after plasma administration. Additionally, there were significant differences in SpO2 (P = 0.000), lymphocyte count (P = 0.000), PLT count (P = 0.005), and ESR (P = 0.007) on different days after CP administration (Table 3).
| Factors | Day 0 of Transfusion | Day 4 of Transfusion | Day 7 of Transfusion | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients | Controls | P-Value | Patients | Controls | P-Value | Patients | Controls | P-Value | |
| Oxygen saturation | 77.27 ± 12.81 | 75.75 ± 14.59 | 0.614 | 88.22 ± 7.00 | 88.80 ± 5.72 | 0.707 | 88.75 ± 6.16 | 88.86 ± 5.74 | 0.934 |
| Lymphocyte | 12.92 ± 7.57 | 12.51 ± 8.98 | 0.817 | 7.92 ± 6.45 | 10.56 ± 9.88 | 0.116 | 8.84 ± 8.11 | 15.18 ± 28.86 | 0.094 |
| Platelet | 206.24 ± 69.50 | 212.12 ± 84.15 | 0.720 | 250.13 ± 133.50 | 235.84 ± 67.94 | 0.607 | 224.77 ± 122.39 | 256.52 ± 114.64 | 0.271 |
| C-reactive protein (mg/L) | 26.83 ± 19.53 | 26.08 ± 19.19 | 0.871 | 25.25 ± 19.89 | 39.00 ± 28.16 | 0.337 | 14.00 ± 8.04 | 160.00 ± 327.66 | 0.257 |
| Erythrocyte sedimentation rate | 54.02 ± 13.83 | 53.00 ± 12.94 | 0.815 | 38.44 ± 19.98 | 41.75 ± 13.02 | 0.696 | 40.75 ± 23.43 | 28.50 ± 27.23 | 0.436 |
| Lactate dehydrogenase (u/i) | 1072.18 ± 574.62 | 1133.90 ± 356.45 | 0.633 | 1299.08 ± 889.86 | 1362.40 ± 616.19 | 0.774 | 2022.13 ± 3202.13 | 1678.64 ± 821.03 | 0.665 |
| D-dimer (mg/L) | 2521.41 ± 2818.56 | 2380.08 ± 2134.20 | 0.871 | - | - | - | 885.00 ± 424.26 | 10000.00 | 0.036 |
a Values are expressed as mean ± standard deviation.
| Factors | Day 0 | Day 4 | P-Value | Day 4 | Day 7 | P-Value |
|---|---|---|---|---|---|---|
| Body temperature | 36.81 ± 0.84 | 36.82 ± 0.45 | 0.915 | 36.82 ± 0.45 | 36.81 ± 0.45 | 0.846 |
| Respiration | - | - | - | 21.16 ± 3.10 | 21.62 ± 2.97 | 0.326 |
| Oxygen saturation | 77.27 ± 12.81 | 88.22 ± 7.00 | 0.000 | 88.22 ± 7.00 | 88.75 ± 6.16 | 0.613 |
| Lymphocyte | 12.92 ± 7.57 | 7.92 ± 6.45 | 0.000 | 7.92 ± 6.45 | 8.84 ± 8.11 | 0.427 |
| Platelet | 206.24 ± 69.50 | 250.13 ± 133.50 | 0.005 | 250.13 ± 133.50 | 224.77 ± 122.39 | 0.207 |
| C-reactive protein | 26.83 ± 19.53 | 25.25 ± 19.89 | 0.794 | 25.25 ± 19.89 | 14.00 ± 8.04 | 0.175 |
| Erythrocyte sedimentation rate | 54.02 ± 13.83 | 38.44 ± 19.98 | 0.007 | 38.44 ± 19.98 | 40.75 ± 23.43 | 0.830 |
| Lactate dehydrogenase | 1072.18 ± 574.62 | 1299.08 ± 889.86 | 0.081 | 1299.08 ± 889.86 | 2022.13 ± 3202.13 | 0.144 |
| Day 0 | ||||||
| D-dimer | 2521.41072818.56310 | 0.009 | 7172.00004898.23968 |
a Values are expressed as mean ± standard deviation.
4.3. Impact of Gender and Age on Laboratory Data of CP-Treated Patients
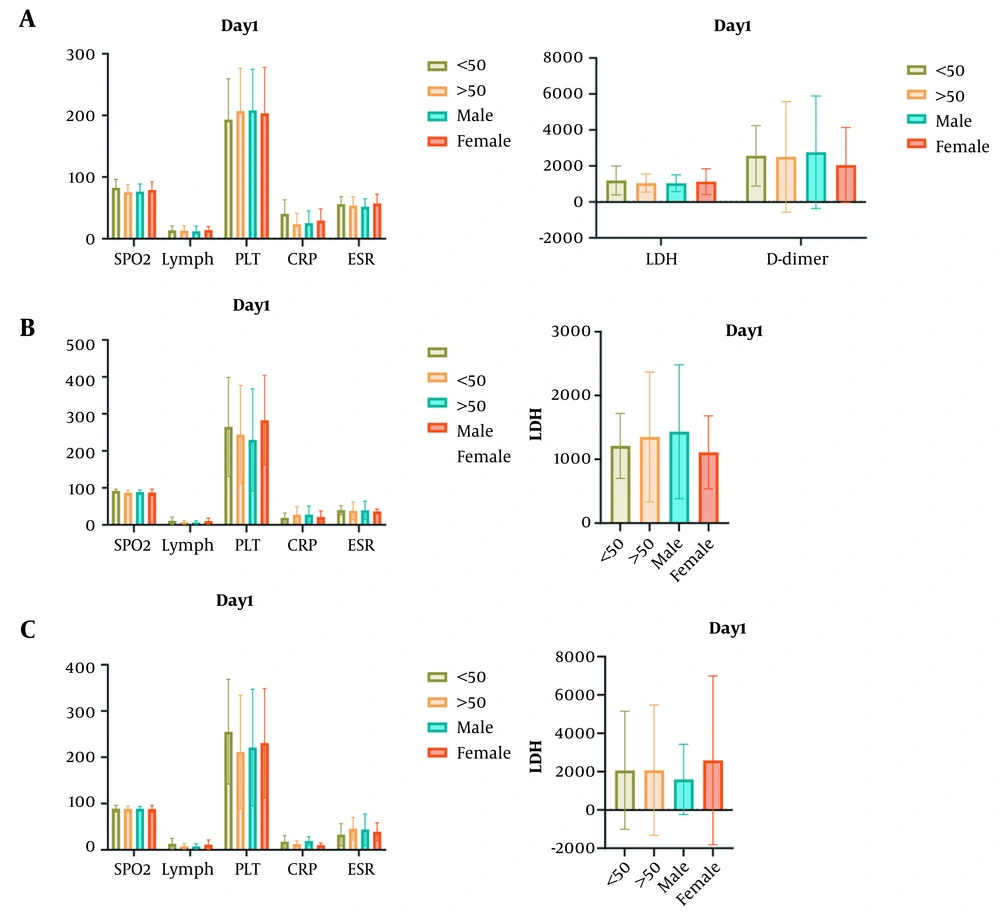
The results showed a significant difference between patients under 50 years and those over 50 years in SpO2 on the fourth day (P = 0.004) and the number of lymphocytes on the fourth and seventh days (P = 0.011 and P = 0.012, respectively) (Figure 1). In addition, a significant difference was observed between male and female subjects in the number of lymphocytes after plasma treatment on the fourth and seventh days (P = 0.006 and P = 0.042, respectively).
Analysis of impact of age and gender on clinical data of coronavirus disease 2019 (COVID-19) patients; A, Comparison of laboratory data of patients on the first day of hospitalization between males and females and age groups ≤ 50 and ≥ 50; B, Comparison of oxygen saturation and lymphocyte count on the 4th day between gender groups indicative of significant changes; C, Changes in lymphocyte count on the 7th day of treatment (SpO2, oxygen saturation; lymph, lymphocyte count; PLT, platelet; CRP, c-reactive protein; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase).
5. Discussion
Passive immunization is a method used to provide short-term immunity to infections by administering specific antibodies against pathogens and has been lifesaving since its earliest use. The plasma of infected patients is one of the primary sources of these specific antibodies and inflammatory cytokines and chelating factors, which have been used to treat patients with viral infections, such as Ebola, SARS, MERS, Junin virus, and Lassa virus. Therefore, the present study investigated the effect of CP infusion on clinical factors in patients with COVID-19 (20, 21).
One of the ways to treat COVID-19 is to use NAb. In this study, CP was used as a source of NAb, and the effects of this type of plasma and NAb on the inflammatory factors in patients were investigated to assess the health status of patients and the relationship between NAbs and inflammatory factors.
In a study conducted on SARS, the mortality rate was reduced in the group receiving plasma compared to the control group (6.3% and 21.9%, respectively; P = 0.08) (20). There was also a decrease in the mortality rate for H1N1 influenza (8). Similarly, the present study’s results revealed a 30% decline in the mortality rate of the patients receiving plasma (CP-treated: 53%, control: 84%). The results also indicated an improvement in patients’ clinical factors.
Studies have demonstrated a decrease in lymphocyte count after CP administration (19). However, the present study’s results showed no significant change in the lymphocyte count in the plasma-receiving group compared to the control group. In a pilot study, an increase was noticed in lymphocyte count (0.65 × 109/L to 0.76 × 109/mL) (18). By comparing different days after receiving plasma, first, a significant decrease in the number of lymphocytes was observed, which finally increased slightly. However, the findings of the current study are inconsistent with the findings of a previous study by Hu et al., which showed a decrease in the number of lymphocytes (22).
Previous studies reported an increase in CRP, LDH, D-dimer, and ESR (19). The CRP is an acute inflammatory protein and a biomarker for inflammation and infections (23), and its increase can be correlated to inflammatory responses (24) that might have been caused by viral infections (25). A study conducted by Sadeghi-Haddad-Zavareh et al. revealed higher CRP levels in patients with severe diseases than in other patients (16). This correlation has also been confirmed in several studies (26, 27). The present study’s findings showed a decrease in the patients’ CRP levels. Similar results were also obtained in a study performed by Brown and McCullough (18) and in another study carried out in a hospital in Shenzhen, China (28). The reduction of CRP levels in patient blood could result from inflammation reduction in response to CP transfusion and an increase in NAbs.
Similar to CRP, ESR is an inflammatory factor that can increase viral infections. In addition, an increase in the level of this factor might be observed in COVID-19, which might contribute to the cytokine storm (14). The present study investigated the patients’ ESR levels to determine the effect of CP therapy on ESR level alterations. The results indicated a significant decrease in ESR levels in patients receiving CP. Therefore, CP transfusion might play an important role in the reduction of cytokine storm in the body (29).
D-dimer is formed from the breakdown of fibrin by plasmin and can be observed in pathological or non-pathological processes, such as arterial thrombosis, pregnancy, inflammation, and cancer. Changes in the amount of fibrin can lead to changes in plasma D-dimer levels (17, 30). Yao et al. performed a study on the D-dimer level in COVID-19 patients and showed a significant elevation in this parameter in 74% of the study population (31). Additionally, the median D-dimer level was higher in nonsurvivors than in survivors (6.21 vs. 1.02 mg/L) (31). Several studies have also proved the correlation between an increased D-dimer level and disease severity (32-34). The present study’s results showed a significant decrease in the D-dimer levels, which suggested that CP treatment could play an effective role in suppressing the D-dimer production process.
The LDH is another biomarker that has been investigated for its role in COVID-19 infection (35). The evidence shows that there are significant differences in the LDH levels of COVID-19 patients (15). The LDH is an intracellular enzyme that catalyzes the reciprocal conversion of pyruvate and lactate (36). It has been used as a marker of cardiac injuries (37). Due to the presence of LDH in the lung tissue, COVID-19 patients might release excessive amounts of this enzyme in circulation. Studies have demonstrated a correlation between LDH levels and worse outcomes among COVID-19 patients (38, 39). The present study’s results showed a decrease in LDH levels in CP-treated patients, which might be a sign of controlling LDH levels by plasma injection and preventing the unpleasant consequences of the disease.
There was no age or gender subgroup analysis in the literature; even one study stated that age was a poor prognostic factor (40). Nevertheless, in the current study, the patients under 50 years showed significant changes in SpO2 and lymphocyte count on the fourth and seventh days after CP transfusion. Additionally, there were significant differences between males and females in the aforementioned factors on the fourth and seventh days after CP treatment. This result shows that in future studies, age and gender should be considered effective factors in the treatment and control of the severity of infection in younger individuals or specific gender.
There were the following limitations in this study:
(1) One week after the CP injection, the patients could not be followed up because most of them died during the treatment;
(2) It was very difficult to find patients based on the inclusion and exclusion criteria;
(3) There were not enough individuals who had recovered from COVID-19 who met the conditions for plasma donation based on this study’s inclusion and exclusion criteria;
(4) It was difficult to find patients with a similar treatment regimen.
In conclusion, this study showed the potential impact of CP therapy on the important clinical parameters of COVID-19, including lymphocyte count, D-dimer, and ESR. However, further comprehensive studies are required to explore the effects of CP on other clinical factors. Overall, plasma therapy can reduce mortality and more severe symptoms. Therefore, in the absence of drugs that can suppress the infection, it can be a good way to control viral infections, such as COVID-19.