1. Background
Respiratory disorders of coronavirus disease 2019 (COVID-19) were first reported in China in December 2019. Initially, the disease was unknown; after investigation, Chinese researchers introduced COVID-19 as the cause of the disease, the scientific name of which is severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) (1). The human-to-human transmission of SARS-CoV2 has been reported through respiratory droplets, direct contact, and sometimes the fecal-oral route. The incubation period is 0 - 24 days, with an average of 3 days (2). The SARS-CoV2 virus can bind to the surface of bronchial epithelial cells and type 2 pneumocytes through its surface receptor (i.e., angiotensin-converting enzyme (2). It infects the cells (as hosts of the virus) and causes respiratory complications in patients (3).
Most patients with COVID-19 show mild to moderate symptoms. Nevertheless, approximately 15% of the cases progress to a severe form, and approximately 5% present with acute symptoms, such as acute respiratory distress syndrome, septic shock, or multiple organ failure (1, 4). A definite laboratory diagnosis of COVID-19 is reverse transcription polymerase chain reaction (RT-PCR). Other laboratory findings include lymphopenia, neutrophilia, elevated liver enzymes, including alanine aminotransferase (ALT) and aspartate aminotransferase (AST), elevated lactate dehydrogenase, C-reactive protein (CRP), ferritin, and D-dimer. The CRP, ferritin, and D-dimer increment are associated with severe disease; lymphopenia and D-dimer increment are also associated with mortality (5).
Among the treatments used for patients with COVID-19, it can be referred to the management of patients’ clinical symptoms and oxygen therapy using a ventilator for patients with respiratory disorders (6). In addition to the above-mentioned treatments, which are based on supportive therapies, drugs can also be used to treat patients with COVID-19 (7). The most important drugs used to treat these patients can be antivirals, antiparasitics, antibiotics, and immunomodulators. Remdesivir, lopinavir, ritonavir, favipiravir, umifenovir, chloroquine, hydroxychloroquine, azithromycin, interferon beta-1, and anakinra are among the drugs that are classified in the above-mentioned drug category and have good efficacy for the treatment of patients with COVID-19 (8).
Studies have shown that in addition to drugs and supportive therapies, monoclonal antibodies and immunotherapy using intravenous immunoglobulin, which can neutralize the virus, are also effective in treating patients with COVID-19. The most widely used and effective monoclonal antibodies in this field are sarilumab, tocilizumab, bamlanivimab, and etesevimab. Dietary supplements, such as vitamin C, can also be used to treat COVID-19 to improve clinical symptoms and strengthen the immune system. Several vaccines can also be effective in treating patients with COVID-19; however, they are still under discussion, and more scientific research is needed to prove their effectiveness in treating the disease (8-10).
In addition to the above-mentioned findings, one of the effective treatment strategies for COVID-19-infected individuals is convalescent plasma therapy (CPT) (11). The extent to which CPT is used to treat patients with COVID-19 varies worldwide. In Iran, the use of this method is reported to be 50% on average; however, in Iraq, Argentina, Australia, Spain, and Saudi Arabia, this rate has been 42.8%, 16%, 28.3%, 46.9%, and 24.3%, respectively (12, 13). Additionally, in a study conducted in Italy, using CPT treatment was reported to be different depending on the dose of convalescent plasma (CP). The rates of using this treatment in individuals consuming 1 unit, 2 units, and 3 units of CP are 68.2%, 27.3%, and 4.5%, respectively (14). In general, the results of studies have shown that using the CPT method reduces the mortality rate in patients consuming CP by 31% (12). The mechanism of CP utilization is the modification of inflammatory responses and the function of neutralized antibodies, which bind to the pathogenic agents and inhibit them; it subsequently leads to the occurrence of the antibody-dependent cellular cytotoxicity complement activation and phagocytosis process. Moreover, the presence of non-neutralizing antibodies binding to the pathogens might also be helpful (15).
Studies have shown the effectiveness of CPT in reducing mortality in patients with influenza A H1N1 virus (16), the Middle East respiratory syndrome, and severe acute respiratory syndrome, which belong to the coronavirus family (17, 18). In addition, the results of studies have shown that the CPT method can be effective in treating patients with COVID-19. In a study conducted in China by Huang et al., the effectiveness of this treatment was reported to be 58.3%. Moreover, in this study, no specific complication was reported due to CP injection, and the lymphocyte count increased to normal after CP injection. In addition, other COVID-19-related test results, such as CRP, ALT, AST, and white blood cells, decreased after CP injection (19).
In another study conducted by Salazar et al. at Houston hospital in Texas, United States, it was shown that in the CPT method, among 25 patients after 14 days of CP injection, 19 patients (76%) had at least one of the symptoms of recovery from the disease according to World Health Organization criteria and 11 patients were discharged from the hospital (20). Furthermore, the results of a study by Mahapatra et al., which was conducted in India, showed that using the CPT method increases the rate of complete recovery in plasma recipients who have a high titer of neutralizing antibodies (21). Therefore, the current study investigated the use of CP in COVID-19 treatment as a treatment strategy.
2. Objectives
This study aimed to evaluate the effectiveness of CPT in the treatment of patients with COVID-19. In order to achieve maximum effectiveness in this method, it is necessary to obtain the appropriate volume of plasma and plasma with a high titer of neutralizing antibody. Therefore, in this study, the plasma of different blood groups and the volume of injected plasma in COVID-19 patients were studied to obtain plasma with a high titer of neutralizing antibody and appropriate volume to maximize the effectiveness of CPT treatment in severe cases.
3. Methods
3.1. Research Design, Population, and Sampling
The present study was performed in Shiraz, Fars province, Iran, within 5 May 2020 to 4 February 2021. The sampling method in this study was random sampling, and the sample size was calculated at 440 subjects based on the following formula:
In this formula, the probability of the first type error is 0.05, and the probability of the second type error is 0.2.
Informed consent was obtained from patients and their relatives. The inclusion criteria were patients aged ≥ 18 years and hospitalized with positive COVID-19 tests and complete clinical information. Pneumonia was confirmed with chest radiographs, severe clinical signs, or life-threatening symptoms, and sequential organ failure assessment (SOFA) or modified SOFA score doubled or greater than the baseline score (the score range of this system: 0 - 24; the higher the value, the more severe the disease status).
The exclusion criteria were lactation or pregnancy, immunoglobulin A deficiency, presence of conditions that increase thromboembolism risk, life expectancy less than 24 hours, severe septic shock, a history of allergic reactions to blood transfusions and related products, disseminated intravascular coagulation, severe congestive heart failure, presence of pneumonia in patients as a result of infectious agents other than SARS-CoV2, and attending other clinical trials 30 days before enrolling in the present study. In addition, clinical information related to patients with COVID-19 was extracted from their medical records, which were approved by a physician. This information includes demographic data (e.g., age and gender), laboratory tests, CP injection dose, number of hospital days, and medical interventions, such as auxiliary mechanical ventilation, nasal oxygen inhalation, and medication scheme.
After complete recovery from COVID-19, 220 patients were called for apheresis and plasma isolation; they were referred to the blood donation centers of Shiraz. Donor selection criteria were the absence of clinical symptoms for at least 7 days, passing at least 3 weeks from the onset of clinical symptoms, two consecutive negative RT-PCR tests at least 24 hours apart, a negative test for other pathogens causing respiratory disorders and hepatitis B virus, hepatitis C virus, human immunodeficiency virus, and syphilis, and neutralized antibody titers within the range of
3.2. Ethical Considerations
This retrospective study was conducted under the auspices of the Ethics Committee of the Iranian Blood Transfusion Organization (IBTO). All procedures performed in this study involving human participants were in accordance with the ethical standards of the IBTO.
3.3. How to Analyze Data
For data analysis, continuous parameters have been reported as mean and standard deviation. Categorical variables have been reported as numbers and percentages. Pearson’s chi-square test was used to evaluate the antibody titer in donors and its relationship with blood groups. IBM SPSS statistical software (version 23.0) was used for all statistical analyses. A P-value less than 0.05 was considered the significant level.
4. Results
4.1. Participants’ Demographic Characteristics
The present study involved 220 CP donors and 220 COVID-19 patients (a total of 440 individuals). The mean age values of plasma donors and COVID-19 patients were 48.9 ± 5.8 and 54.3 ± 15.7 years, respectively. Among the plasma donors, 17 and 203 subjects were female and male, respectively. The mean age values of female and male participants were 47.2 ± 5.6 and 49.1 ± 5.8 years, respectively. Among the CP recipients, 88 and 132 cases were female and male, respectively; the mean age values were also 56.1 ± 17.2 and 53.1 ± 14.6 years, respectively (Table 1).
| Plasma Donors (n = 220) | |
|---|---|
| Age (y) | 48.9 ± 5.8 |
| Gender | |
| Female | 17 (7.73) |
| Male | 203 (92.27) |
| Age (y) | |
| Female | 47.2 ± 5.6 |
| Male | 49.1 ± 5.8 |
| COVID-19 Patients (n = 220) | |
| Age (y) | 54.3 ± 15.7 |
| Gender | |
| Female | 88 (40) |
| Male | 132 (60) |
| Age (y) | |
| Female | 56.1 ± 17.2 |
| Male | 53.1 ± 14.6 |
Abbreviations: COVID-19, coronavirus disease 2019; SD, standard deviation.
a Values are expressed as mean ± SD or No. (%).
Plasma donors were divided into three categories, namely first-time, experienced, and continuous donors, regarding blood donation frequency (Table 2).
| Category of Donor | No. (%) (n = 220) |
|---|---|
| First-time | 77 (35) |
| Experienced | 60 (27.3) |
| Continuous | 83 (37.7) |
4.2. Blood Group
Patients in both groups of donors and recipients were evaluated for the blood group. Among the female donors, 10 and 7 individuals belonged to B+ and O+ blood groups, respectively. However, none of the patients belonged to A+, AB+, and O- blood groups. Among male donors, 24, 9, 51, 107, and 12 participants belonged to A+, AB+, B+, O+, and O- blood groups, respectively (Table 3).
| Blood Group | Plasma Donors (n = 220) No. (%) |
|---|---|
| A+ | |
| Female | 0 |
| Male | 24 (10.9) |
| AB+ | |
| Female | 0 |
| Male | 9 (4.09) |
| B+ | |
| Female | 10 (4.5) |
| Male | 51 (23.1) |
| O+ | |
| Female | 7 (3.1) |
| Male | 107 (48.6) |
| O- | |
| Female | 0 |
| Male | 12 (5.45) |
4.3. Antibody Titration
The antibody titers of CP donors were analyzed according to their blood groups. In individuals with blood group A (A+), 21 and 3 cases had titers of
| Blood Group | Antibody Titers of Plasma Donors (n = 220) No. (%) | ||||
|---|---|---|---|---|---|
| A+ | 0 | 21 (9.54) | 0 | 0 | 3 (1.3) |
| AB+ | 0 | 0 | 9 (4.09) | 0 | 0 |
| B+ | 26 (28.1) | 25 (11.3) | 10 (4.54) | 0 | 0 |
| O+ | 0 | 0 | 4 (1.81) | 91 (41.3) | 19 (8.6) |
| O- | 0 | 0 | 12 (5.45) | 0 | 0 |
a Antibody titration was performed semi-quantitatively. Therefore, the donor plasma was diluted to the ratios of
The median antibody titer was
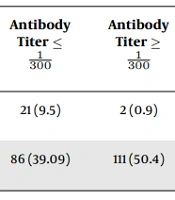
Statistical analysis showed that most expired patients received plasma from donors who had titers of ≤
| To Be Revived | Antibody Titer ≤ | Antibody Titer ≥ | Odds Ratio (Confidence Interval) | P-Value b | Rate of Injected Plasma Less than 387 mL | Rate of Injected Plasma Greater than 387 mL | Odds Ratio (Confidence Interval) | P-Value b |
|---|---|---|---|---|---|---|---|---|
| Expired | 21 (9.5) | 2 (0.9) | 0.08 (0.009 - 0.36) | < 0.001 | 17 (7.7) | 6 (2.7) | 0.3 (0.91 - 010) | 0.018 |
| Discharged | 86 (39.09) | 111 (50.4) | 1.5 (1.06 - 2.3) | 0.01 | 54 (24.5) | 143 (65) | 5.7 (3.7 - 8.8) | < 0.001 |
a Values are expressed as No. (%).
b Significance level is less than 0.05.
5. Discussion
The CP as a treatment modality has been used for many years (22). Antibodies in plasma create passive immunity by binding and neutralizing the pathogen (23). According to the results of previous studies, CP has been currently considered a treatment for COVID-19-infected individuals (24). Using CP is very effective in resuscitating patients and improving their clinical conditions (13).
Previous studies have shown the importance of blood groups in COVID-19 patients. In addition, antibody titers vary in different blood groups. A study by Bloch et al. showed that antibody titers were higher in individuals with the B blood group than in other blood groups, which was statistically significant (25). However, Gallian et al.’s study showed that antibody titers were lower in individuals with the O blood group than in others (26). In the present study, the antibody titer was higher in individuals with the O+ blood group than in others. Therefore, blood group O could play a good protective role, compared to other blood groups, against COVID-19 by increasing antibody production.
There are numerous debates over which blood type has the highest antibody titer. However, since patients with the O blood group have been shown to be less susceptible to COVID-19, it shows higher antibody titer in these patients; nevertheless, it requires further research. Studies reported a direct relationship between antibody titer levels in plasma and the recovery rate of COVID-19 patients. To this end, Joyner et al.’s study showed that increasing the antibody titer in plasma donors could improve patients’ clinical status and reduce COVID-19-induced mortality (27).
Furthermore, a study by Libster et al. showed that a high antibody titer plasma injection into patients in the early stages of the disease prevents disease progression and leads to increased survival (28). In the present study, antibody titer increment caused patients’ discharge. Most patients who were recovered and discharged received plasma from high-titrated donors. Most patients who expired received plasma from low-titrated donors.
In addition, it was shown that there was a significant relationship between the plasma transfusion rate and patient discharge. In other words, the number of patients who received more than 387 mL plasma was higher in terms of discharge than patients who received less than 387 mL. Most of the patients who received less than 387 mL CP were expired. This might be due to an increase in the level of antibodies in the injected plasma. Therefore, in addition to antibody titer, plasma volume can also be effective in the CPT method. Therefore, the level of antibody titers in donor plasma can have a great impact on the survival of blood recipients, which can be widely studied in the future.
5.1. Limitations
Although this study examined the plasma of different blood groups to obtain plasma with neutralizing antibody titer and appropriate volume to maximize the effect of the CPT method, which is the strength of this study, it has some limitations. These limitations include following up on patients, using a statistical population with a larger sample size, and comparing the CPT method to other treatments used in COVID-19 to achieve the most appropriate treatment.
5.2. Conclusions
In this study, it was shown that patients with COVID-19 who received plasma with a titer higher than