1. Background
Schizophrenia is recognized as one of the twenty leading causes of disability worldwide. This disorder has significant health, social, occupational, and economic consequences. These consequences can be the result of the early onset of the disorder and its severe and persistent symptoms (1). The findings of Shinn and Viron suggest that schizophrenia is associated with cognitive impairments, including executive dysfunction. In addition, individuals with schizophrenia, who are stigmatized by society, have insufficient insight (2). Insight refers to a person’s ability to diagnose (psychological insight) and accept mental illness (emotional insight). Therefore, a lack of insight means a lack of awareness of one’s mental illness (3). However, impaired insight is more commonly seen as a hallmark of schizophrenia. A significant proportion of patients with schizophrenia have poor or no insight. Accurate knowledge of the patient’s level of insight can help us gain access to the pathology of the disorder, its prognosis, and aspects of its treatment (4). Inadequate insight or lack of insight into the disease is a major barrier to treating individuals with schizophrenia. However, giving insight to some individuals with the disease will lead to stability and improvement (5).
What is certain, however, is that poor insight prevents the treatment of psychosis in the early stages of the disorder (6). Poor insight into the disease can lead to partial adherence or non-adherence to treatment in a large number of patients with schizophrenia. Non-adherence to treatment is a major risk factor for poor treatment outcomes, such as relapse, readmission, and suicide (7). Poor medication adherence is a major factor in the recurrence of the disease in individuals with mental disorders. Therefore, supporting drug adherence is an important issue in the recovery and rehabilitation of patients with mental disorders (8). Based on the evidence, 41 - 53% of patients with schizophrenia have poor drug adherence (9). However, drug adherence factors in patients with schizophrenia have always been crucial in the treatment and rehabilitation of these patients. However, treatments are not the same in different countries, and no study has been conducted on the factors affecting drug adherence in different mental healthcare settings (10).
Although pharmacotherapy has been the mainstay of treatment for patients with schizophrenia since the first half of the twentieth century, whether or not a patient accepts medication is crucial in clinical decision-making because it plays an important role in the effectiveness and adherence to treatment (11). However, despite their effectiveness, a total of 37.5% of the patients on oral antipsychotics and 11.5% of those on long-acting injectables abandoned the treatment (12).
Accordingly, in recent decades, psychiatry has not only alleviated the symptoms of mental disorders but is also related to the quality of life of these patients, their return to previous functioning, and their well-being. That is, to change the paradigm that has transformed research results into mental illness (13). Although the evidence for the effectiveness of psychological interventions for schizophrenia (psychotic disorders in general) is increasing, there is still no consensus on the reduction of symptoms by this type of intervention (14).
Cognitive behavioral therapy (CBT) has been shown to be effective for a wide range of problems, including depression, anxiety disorders, alcohol and drug abuse, marital problems, eating disorders, and severe mental illness. Numerous research studies have shown that this approach leads to a significant improvement in performance and quality of life. Many studies have shown that CBT is as effective as or even more effective than other psychological therapies or psychiatric medications (15). This treatment for psychosis, along with antipsychotic drugs and routine comprehensive care in the management of schizophrenia, is also recommended (16). Several international protocols have also recommended and emphasized this treatment approach to schizophrenia as the gold and standard treatment to meet the needs of these patients (17).
More than 60 randomized controlled trials (RCTs) have examined the effect of CBT on patients with schizophrenia and other psychotic disorders. Although meta-analyses have reported a reduction in a wide range of symptoms, the effect of this therapeutic approach on patients in the early stages of psychosis has been less studied in RCTs. This is important because patients in the early stages of psychosis might have completely different treatment needs than patients with multiple periods and a more prolonged history of illness (18). However, the average size of the effects reported in recent studies is small and even smaller in studies with more rigorous methodologies (19).
Although the effectiveness of CBT in schizophrenia has been somewhat proven, there is less evidence for the group form of this therapeutic approach. For example, in a study by Barrowclough et al., the main hypothesis (i.e., the significant improvement of positive symptoms of psychiatric patients by group cognitive behavioral therapy (GCBT) compared to the usual treatment of patients) was not confirmed by the research findings. However, although there was no significant difference between the intervention and control groups in the severity of symptoms or performance or recurrence, the members of the GCBT group reported a decrease in feelings of hopelessness and low self-esteem (20). However, there are limited empirical studies on the effectiveness of group interventions in individuals with psychosis. The data show that those who are referred to the group intervention program might improve their performance effectively (21). A clinical trial by Elwyn et al. with a very small sample of five subjects showed that the participants were very satisfied with CBT in the form of group intervention, and their psychotic symptoms were significantly reduced after the group intervention compared to those before the intervention (22). Generally, some research results confirm that adding a brief psychological intervention to routine care in a psychiatric clinic is an effective way to improve the significant symptoms of schizophrenia (23).
It seems that taking therapeutic measures in a group manner for patients with schizophrenia is crucial. Since group interventions are more cost-effective, their effectiveness should be analyzed in different cases of mental disorders. Moreover, such interventions can lead to improvements in the treatment of mental disorders. The results of this study can be used in outpatient and inpatient treatment centers to help patients with schizophrenia.
2. Objectives
This study aimed to evaluate the effect of GCBT on the improvement of insight and treatment adherence in patients with schizophrenia.
3. Methods
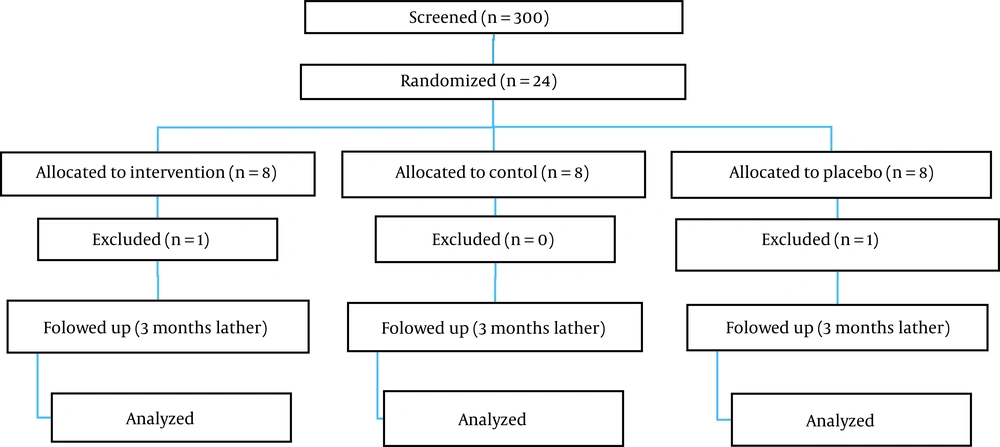
In this study, which lasted from October 2021 to January 2022 in Shiraz Psychiatric Hospital, Shiraz, Iran, 22 eligible participants were placed in the intervention (n = 7), therapist’s attention placebo (n = 7), and control (n = 8) groups via simple randomization.
3.1. Procedure
The data were collected using the Insight and Treatment Attitudes Questionnaire (ITAQ) and the Medication Adherence Rating Scale (MARS) in three stages before, after, and 3 months after the intervention. After examining the patients’ files, volunteers who met the criteria for entering the research were selected. The inclusion criteria were meeting the diagnostic criteria of schizophrenia spectrum disorders according to the diagnostic criteria of the diagnostic and statistical manual of mental disorders-fifth edition, an age range of 18 - 50 years, at least a third grade of middle school education, not receiving electroshock therapy (ECT) for at least 2 months before hospitalization and during hospitalization, and not receiving psychological treatments at the same time as entering the research or 3 months before entering the research. The exclusion criteria were being in the acute phase of the disease, drug abuse, diagnosis of borderline personality disorder, serious suicidal thoughts, any type of severe medical illness that prevents the continuation of treatment, mental retardation, brain damage, more than one session of absence from the group, comorbidity of substance dependence disorder, or comorbidity of other psychiatric disorders.
In the intervention group, the treatment program was carried out by an experienced group therapist during seven 90-minute sessions with 15-minute rest (three times a week) based on the treatment protocol adapted from Robert Paul Lieberman’s 16-session community return program. Each session started with a preliminary discussion and questions. Then, the discussion continued for one and a half hours on the topic of the same session. According to the goals of each session, exercises were done in the form of role-playing, checking the relevant checklist, or expressing their experiences to other members of the group. Moreover, at the end of each session, the summarized information about the same session was given to the patients in the form of brochures. The meeting ended by evaluating the level of activity and participation in group activities. At the beginning of the next session, a summary of the previous session was presented by the group members, and the necessary corrective information was provided to the participants through the therapist. In order to investigate the effect of the therapist’s attention, the placebo group of the therapist’s attention watched documentary films with no psychological background for seven sessions and discussed the topic of the film and its scenes. The control group also received only their usual treatments, which included meeting with a psychiatrist. All three interventions, therapist’s attention placebo, and control groups completed the relevant tests again after the completion of the intervention and 3 months after the intervention.
Although participation in the study was optional, written informed consent was obtained from the participants. Ethical approval was obtained from the Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran (IR.SUMS.REC.1400.673), and the study was registered at the Iranian Registry of Clinical Trials (IRCT ID: IRCT20210912052449N1).
The files of patients who visited the hospital during the 4-month period from October 2021 to January 2022 were reviewed. If the patients met the criteria for entering the research, the goals of the project were explained to them, and if they agreed to participate in the project, they were referred to a clinical psychologist. By providing the informed consent form, the clinical psychologist entered the patient as a research sample into the evaluation and treatment process (Figure 1). Evaluations in all stages of the research were carried out by a researcher with a master’s degree in clinical psychology, other than the therapist. The subjects were randomly assigned to three groups, namely CBT, therapist’s attention placebo, and control. All the patients received their usual treatments during hospitalization, according to the opinion of the attending psychiatrist. In addition, the patients in all three groups were matched regarding the number of hospitalizations, class of drugs, and receiving ECT. In the intervention group, the treatment program was performed in seven 90-minute sessions based on a treatment protocol adapted from Robert Paul Lieberman’s 16-session community return program. Table 1 shows the main format of this program. The therapist’s placebo group only watched documentaries for seven sessions aiming at neutralizing the therapist’s attention to determine the effect of the treatment program apart from the role of the therapist’s attention. The control group received only their usual treatments, which included a visit to a psychiatrist. Then, all the patients completed the research tools, which included the questionnaires of the ITAQ and MARS.
| Session | Title | Worksheet | In-session Practice | Brochure |
|---|---|---|---|---|
| 1 | The nature of mental disorders, stigmatization, and normalization | Knowledge of disease | Expressing experiences to other group members | Schizophrenia in plain language |
| 2 | Communication skills and expressing feelings | Effective factors in a good communication | Role-play | Communication skills in plain language |
| 3 | Symptoms of the disorder and relapse symptoms | Symptoms of my disease | Diagnosis of symptoms based on the checklist | Summary of important symptoms of the disorder |
| 4 | Drug treatment benefits and side effects | The names of drugs and their side effects | Drug experience report | Benefits of the drug |
| 5 | Stress management skills | List of my stressors | Role-play | Facing stress in society |
| 6 | Rehabilitation and self-care skills | Factors to improve my acceptance in society | Role-play | Self-care |
| 7 | Preparation for discharge | Daily schedule | Role-play | Prevention of relapse |
Summary of Group Cognitive Behavioral Therapy for Schizophrenia Patients
3.2. Instruments
Sociodemographic information, the ITAQ, and the MARS were used in this study. The ITAQ is an 11-item semi-structured interview designed to assess the patient’s awareness of psychiatric illness, especially schizophrenia, and the need for treatment. Each item is scored between 0 and 2. The maximum score on this scale is 22. The score ranges of 0 - 7, 8 - 14, and 15 - 22 indicate poor, average, and good insight, respectively. Principal component analysis extracted a single factor for the ITAQ. Correlations with an open interview methodology supported construct validity. Medication compliance and ITAQ scores were moderately inversely correlated at initial and day 14 assessments, further supporting construct validity. Scores were moderately inversely correlated with Brief Psychiatric Rating Scale and clinical global impressions scores. However, overall, there was no consistent relationship between changes in psychopathology and changes in insight over time (24). The MARS also includes 10 items with yes and no answers that can be easily answered by the patient or the therapist. This scale was prepared by Thompson et al. to simply evaluate patient medication compliance (25). The reliability of this scale has been reported by Cooke in Turkey as satisfactory. The score ranges of 0 - 7 and 8 - 10 indicate weak/low and high cooperation, respectively. It can be said that the MARS is a reliable scale (26). In a study by Javadpour et al., the reliability coefficient obtained from the retest method was reported as 0.91 (27).
3.3. Data Analysis
Descriptive statistics are expressed as mean and standard deviation (SD) in all groups. Moreover, repeated measures analysis of variance (ANOVA) and one-way ANOVA between groups were applied to determine the effect of the GCBT program on insight and medication adherence. Additionally, the significance level of P < 0.01 was used to investigate research findings.
4. Results
The average age of the intervention group was 37 years. However, 42 and 43 years were reported as the average ages for the placebo and control groups, respectively. In order to analyze the research hypotheses, Table 2 shows descriptive and inferential statistics as mean and SD for variables. Repeated measures ANOVA and one-way ANOVA between groups were conducted for both variables separately. Tables 3 and 4 show the obtained results.
| Variables | ITAQ | MARS |
|---|---|---|
| Pre-test | ||
| Experiment | 5.71 ± 1.38 | 4.14 ± 1.21 |
| Placebo | 5.38 ± 1.3 | 3.88 ± 0.99 |
| Control | 5 ± 1 | 4.29 ± 1.11 |
| Post-test | ||
| Experiment | 11 ± 1.63 | 7.14 ± 1.34 |
| Placebo | 5.50 ± 1.92 | 4.63 ± 1.18 |
| Control | 5.71 ± 1.11 | 5 ± 1.29 |
| Follow-up (3 months) | ||
| Experiment | 9.29 ± 1.11 | 7.43 ± 1.13 |
| Placebo | 4.50 ± 1.77 | 3.62 ± 1.06 |
| Control | 4.43 ± 0.97 | 5.14 ± 1.96 |
Mean and Standard Deviation of Dependent Variables in Three Study Groups a
| Groups | Intervention Group | Placebo Group | Control Group | |||
|---|---|---|---|---|---|---|
| ITAQ | MARS | ITAQ | MARS | ITAQ | MARS | |
| N | 7 | 7 | 7 | 7 | 8 | 8 |
| F | 380.455 | 160.72 | 2.519 | 3.923 | 2.726 | 2.721 |
| Sig. | 0.001 | 0.001 | 0.161 | 0.081 | 0.158 | 0.159 |
| Effect size | 0.993 | 0.985 | 0.456 | 0.567 | 0.522 | 0.521 |
Comparison of Baseline, Post-intervention, and 3-Month Follow-up Scores of Insight and Medication Adherence in Three Study Groups
| Variables | Pre-test | Post-test | 3-Month Follow-up | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Between Groups | Within Groups | Total | Between Groups | Within Groups | Total | Between Groups | Within Groups | Total | |
| Sum of squares | |||||||||
| ITAQ | 1.787 | 29.304 | 31.091 | 139.344 | 49.429 | 188.773 | 110.857 | 35.143 | 146 |
| MARS | 0.657 | 23.161 | 23.818 | 26.722 | 30.732 | 57.455 | 54.326 | 38.446 | 92.773 |
| df | |||||||||
| ITAQ | 2 | 19 | 21 | 2 | 19 | 21 | 2 | 19 | 21 |
| MARS | 2 | 19 | 21 | 2 | 19 | 21 | 2 | 19 | 21 |
| Mean square | |||||||||
| ITAQ | 0.894 | 1.542 | 69.672 | 2.602 | 55.429 | 1.850 | |||
| MARS | 0.329 | 1.219 | 13.361 | 1.617 | 27.163 | 2.023 | |||
| F | |||||||||
| ITAQ | 0.579 | 26.781 | 29.967 | ||||||
| MARS | 0.270 | 8.260 | 13.424 | ||||||
| Sig. | |||||||||
| ITAQ | 0.75 | 0.001 | 0.001 | ||||||
| MARS | 0.766 | 0.003 | 0.001 | ||||||
| Eta squared | |||||||||
| ITAQ | 0.057 | 0.738 | 0.759 | ||||||
| MARS | 0.027 | 0.465 | 0.585 | ||||||
Comparison of All Groups’ Baseline, Post-intervention, and 3-Month Follow-up Scores of Insight and Medication Adherence
Insight and medication adherence at the baseline were not different in the three groups (Table 2). The results of repeated measures ANOVA revealed that insight and medication adherence only significantly differed in the intervention group (with the mean scores of the baseline, post-intervention, and 3 months after intervention as 5.71 ± 1.38, 11 ± 1.63, and 9.29 ± 1.11, respectively; P < 0.001). There was no significant difference in the mean scores of the baseline (5.38 ± 1.3 and 5 ± 1), post-intervention (5.50 ± 1.92 and 5.71 ± 1.11), and 3 months after intervention (4.50 ± 1.77 and 4.43 ± 0.97) regarding insight (P < 0.161 and P < 0.157) and medication adherence (P < 0.081 and P < 0.159) between the placebo and control groups, respectively (Table 3).
The results of one-way ANOVA between groups (Table 4) also indicated no significant difference between all three groups in the pre-test. Nevertheless, there was a significant difference between the intervention group with the placebo and control groups in the post-test and follow-up stages (P < 0.001). However, no significant difference was observed between the placebo and control groups.
5. Discussion
The present study aimed to investigate the role of GCBT in insight and medication adherence in patients with schizophrenia in Shiraz. The research findings indicated that GCBT influenced insight and medication adherence in patients with schizophrenia. Three groups were balanced at the baseline, and all variables were not significantly different at the baseline. At the post-intervention stage, insight and medication adherence significantly improved only in the intervention group, and this difference was maintained 3 months after the intervention. The current findings suggest that GCBT might be beneficial in increasing insight and medication adherence in patients with schizophrenia.
A study conducted by Ran et al. supports the findings of the present study. They demonstrated that treatment adherence in the experimental group was significantly higher than in other groups (28). The findings of Baruah and Reddemma’s study are also in line with the results of the current study. The aforementioned study indicated a significant difference between pre-test and post-test in medication adherence scores in the study group. Additionally, it was shown that regarding medication adherence, there was a significant difference in post-test scores between the control and study groups. This observation indicated better gain in comparison to the control group and the efficacy of GCBT in schizophrenic patients (29). Moreover, the results of another study on medication adherence by Chaiyajan et al. are nearly similar to the present study’s results, and the difference in the proportion of participants was statistically significant regarding adherence (30). According to Weiden et al.’s findings, although schizophrenic patients who underwent CBT did not establish a good therapeutic alliance, they used drugs more than other patients (31).
Many studies have also confirmed the effect of GCBT on insight. The importance of providing information and cognitive-behavioral strategies in the management of patients with psychotic symptoms has been highlighted in the literature. It is clear that providing sufficient information and using CBT strategies lead to an increase in patient perception of the disease and help improve insight and adherence to medication. The findings of Al-yahya’s research showed that psychological intervention had an effective role in improving insight and medication adherence for most patients with schizophrenia (32). The evidence supports the role of insight in psychotherapy outcomes. Insight might be a mechanism for change in various therapeutic approaches. Greater insight is moderately associated with better psychotherapy outcomes.
The lack of medication adherence is common among schizophrenic patients due to various factors, including lack of insight, side effects of drug treatments, stigma, inadequate care, cultural influences, and socioeconomic status. Among these patients, non-adherence to trial treatment is problematic, as it can lead to worsening of symptoms, relapse, readmission or greater use of emergency psychiatric services, decreased function, and increased risk of death (33).
Because CBT can improve medication adherence and disorder symptoms, this approach offers a more appropriate healthcare opportunity than information-based education alone. One of the main challenges for medication adherence based on CBT is the induction of unwanted (and often biased) assumptions and thoughts in patients. Therefore, building trust is the basis for providing patients with pharmacological support based on CBT. Therefore, it can be said that CBT can help patients learn effective self-help skills (34).
In psychological interventions for psychotic patients, it can be said that the goal of GCBT is to reduce the discomfort caused by psychotic experiences or at least to enable the patient to deal with this discomfort instead of treating the patient completely. Accordingly, the current study aimed to help patients with schizophrenia in two dimensions, namely strengthening the patient’s insight and improving his/her adherence to drug treatment. This study hypothesized that improved insight through GCBT might increase adherence to drug treatment and vice versa. However, it is more likely that enhancing insight should be the goal of GCBT to improve medication adherence.
It can be said that probably due to the non-compliance of schizophrenic patients with drug treatment, the drug approach alone is not very effective in the treatment of this disorder; nevertheless, it is more effective in combination with psychological interventions, especially CBT. Therefore, the combination of pharmacological interventions with CBT can improve adherence to pharmacological treatment. One of the main reasons for this effectiveness is the patient’s active participation in the treatment process. This active participation can also lead to the creation of a therapeutic alliance. In any case, intervention strategies should be aimed at the patient and his/her support systems to increase the chance of using the medicine. Finally, in this study, GCBT was implemented as a trial in a psychiatric hospital, and the feasibility of implementing the intervention in a limited psychiatric primary care setting was evaluated. Therefore, future evaluations should assess GCBT resources in other treatment settings. However, future research is needed to investigate the possible benefits of GCBT and examine whether the use of this approach can improve patient engagement with psychiatric treatments and increase adherence to pharmacological interventions. Therefore, it should be considered that GCBT can be used alongside drug treatments as a supplemental treatment for patients resistant to antipsychotic treatment.
Cognitive behavioral therapy helps patients learn how to identify and change their destructive thought patterns that negatively affect their behaviors and emotions. In GCBT for psychotic patients, the treatment focuses on changing the negative automatic thoughts of psychotic patients. These thoughts can lead to increased patient resistance to taking medication and following the recommendations of their therapist. Therefore, considering that drug treatments are the first line in the treatment of schizophrenic patients, the results of the present study indicated the need to pay more attention to the use of cognitive behavioral interventions, education, and prevention programs to increase adherence to drug treatments.
5.1. Limitations
In this study, the questionnaires were used to evaluate patients’ insight and treatment compliance. As a result, some patients might refuse to give truthful answers. In addition, the sample size was small; therefore, caution should be exercised in generalizing these findings.
5.2. Conclusions
Insight into the disease and the patient’s adherence to drug treatments have a significant effect on improving the symptoms of schizophrenia patients. As a critical issue, paying attention to the level of insight and treatment compliance of schizophrenic patients is considered an important part of the treatment of these patients. According to this study’s results, GCBT can be effective in the acceptance of the disease and adherence to treatment. Taking into account the beneficial effect of GCBT on insight and medication adherence, it was recommended that GCBT be widely used as a psychological method to improve psychological problems in patients with schizophrenia.