1. Background
Hepatitis B virus (HBV) is one of the most common infections worldwide (1). This liver disease is one of the most important global public health issues today because it is very common in many parts of the world and often leads to chronic cirrhosis and liver cancer (2). Previous estimates have shown that approximately 0.7 to 1.4 million persons in the US are infected with chronic HBV (3, 4). Global Burden of Disease Studies estimates that HBV has caused approximately 786,000 deaths worldwide, mostly due to liver cancer and cirrhosis of the liver (5). HBV infection ranks 15th among all causes of death in humans (6). The global prevalence of HBV varies geographically, from less than 2% in less endemic areas to more than 8% in highly endemic areas (7). According to the CDC and the World Health Organization, Iran and other Middle Eastern countries are moderate endemic areas with a prevalence rate of 2 - 7% (8). HBV can be transmitted from human to human through exposure to contaminated blood and body fluids, especially semen and vaginal secretions (9). The risk of HBV in infants born from mothers with HBsAg+ and HBeAg+ is more than 90 - 70%, with more than 85% becoming chronic carriers of the disease (10).
The hepatitis C virus (HCV), discovered in 1989, is the leading cause of cirrhosis and hepatocellular carcinoma (HCC) (11). Blood transfusions, drug injections, sexual intercourse, and syringes and needles in healthcare settings are the most common routes of HCV transmission (12, 13). Egypt has the highest estimated prevalence globally, followed by India, Pakistan, China, and Indonesia. However, the rates are significantly lower in Australia, Western and Northern Europe, Japan, and North America (14). The prevalence of HCV in blood donors in Iran has been different, and, in a meta-analysis study conducted from 1996 to 2011, it has been shown that out of a total of 10,739,221 blood donors, the prevalence of HCV is 0.5% (15). The epidemiology of HCV in Iran has not been determined precisely. Still, due to the large border between Iran and Afghanistan, as one of the largest producers of opioids in the world, and easy access to these substances, it can be predicted that the prevalence of HCV can be increasing (16, 17).
Identifying epidemiological variables and indicators of HBV and HCV can help implement prevention, control, and treatment programs for health centers and the country (Ministry of Health and Medical Education).
2. Objectives
This research was designed to investigate the change in the epidemiology pattern of HBV and HCV in the south of Fars province from 2015 to 2021.
3. Methods
3.1. Nature of Research
The current research is a cross-sectional study designed in 2021.
3.2. Study Group
The study group consisted of all individuals infected with HBV and HCV between 2015 and 2021 and whose information was available.
3.3. Data Collection
To gather the information, we used a checklist that included data about marital status, gender, age, number of patients by year and month, nationality, living place, and clinical presentations of people with HBV and HCV. Through passive surveillance and referring to the Infectious Diseases Management and Control Center of Larestan Faculty of Medical Sciences, the researchers collected data on HBV and HCV in four cities in the south of Fars province, including Larestan, Evaz, Khonj, and Juyom. After laboratory confirmation, the information of people infected with HBV and HCV was registered in the communicable diseases system of the Ministry of Health in the hepatitis section, and from 2015 to 2021, the researcher gathered the recorded data of these four cities. All health records of patients infected with HBV and HCV were reviewed. The inclusion criterion was the completeness of patient file information, and the exclusion criterion included incomplete patient file information. In this study, in case of incomplete information in the patients' files, after obtaining official permits from the ethics committee and related organizations, additional information was obtained by phone calls to patients whose files were incomplete.
3.4. Statistical Analysis
After collecting the data, we used the statistical software SPSS version 25. The chi-square test was used in each step according to the scale of the data. The Cochran-Armitage trend test was used to determine the incidence of HBV and HCV. In this research, a type 1 error rate of 0.05 was considered.
3.5. Ethical Considerations
To collect the patients' information, necessary arrangements were made with the relevant authorities, and the participants’ information remained confidential throughout the study. All methods and implementation of the study were carried out following the Declaration of Helsinki. The present study was conducted as a part of research project No. 1399-71, which was approved by the Larestan University of Medical Sciences with the Code of Ethics IR.LARUMS.REC.1400.008.
4. Results
In general, in the period from 2015 to mid-2021, a total of 306 cases of HBV and 128 cases of HCV were registered. The mean age of the individuals with HBV was 40.15 ± 18.95 years, and most cases of HBV were reported at 28.1 and 24.9% in the age groups of 50 years or older and 20 - 29 years, respectively. Out of all the cases, 53.6% were women, and 45.1% of those women were of Afghan nationality. Regarding marital status, married people accounted for 83.3% of the cases, and 55.9% lived in rural areas. The results of the chi-square test in different subgroups and by year showed major changes in the incidence of the disease. In all studied variables, a significant difference was observed in different years (P < 0.05) (Table 1).
| Variables | Total | Year of Occurrence | P-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | |||
| Age, (y) | 0.024 a | ||||||||
| 0 - 9 | 5 (1.6) | (0.0) | 0 (0.0) | 1 (20.0) | 2 (40.0) | 1 (20.0) | 1 (20.0) | 0 (0.0) | |
| 10 - 19 | 26 (8.5) | 1 (3.8) | 0 (0.0) | 1 (3.8) | 10 (38.5) | 6 (23.1) | 7 (26.9) | 1 (3.8) | |
| 20 - 29 | 76 (24.9) | 5 (6.6) | 2 (2.6) | 15 (19.7) | 21 (27.6) | 22 (28.9) | 9 (11.8) | 2 (2.6) | |
| 30 - 39 | 59 (19.2) | 2 (3.4) | 0 (0.0) | 6 (10.2) | 12 (20.3) | 26 (44.1) | 10 (16.9) | 3 (5.1) | |
| 40 - 49 | 54 (17.7) | 2 (3.7) | 1 (1.9) | 4 (7.4) | 10 (18.5) | 33 (61.1) | 4 (7.4) | 0 (0.0) | |
| 50 ≤ | 86 (28.1) | 5 (5.8) | 1 (1.2) | 7 (8.1) | 16 (18.6) | 51 (59.3) | 6 (7.0) | 0 (0.0) | |
| Gender | < 0.001 a | ||||||||
| Male | 142 (46.4) | 11 (7.7) | 2 (1.4) | 16 (11.3) | 28 (19.7) | 77 (54.2) | 8 (5.6) | 0 (0.0) | |
| Female | 164 (53.6) | 7 (2.4) | 2 (1.2) | 18 (11.0) | 43 (26.2) | 62 (37.8) | 29 (17.7) | 6 (3.7) | |
| Nationality | 0.033 a | ||||||||
| Iranian | 168 (54.9) | 12 (7.1) | 2 (1.2) | 18 (10.7) | 37 (22.0) | 84 (50.0) | 14 (8.3) | 1 (0.6) | |
| Afghan | 138 (45.1) | 3 (2.2) | 2 (1.4) | 16 (11.6) | 34 (24.6) | 55 (39.9) | 23 (16.7) | 5 (3.6) | |
| Marital Status | 0.024 a | ||||||||
| Single | 51 (16.7) | 6 (11.8) | 2 (3.9) | 4 (7.8) | 16 (31.4) | 17 (33.3) | 6 (11.8) | 0 (0.0) | |
| Married | 255 (83.3) | 9 (3.5) | 2 (0.8) | 30 (11.8) | 55 (21.6) | 122 (47.8) | 31 (12.2) | 6 (2.4) | |
| Place | < 0.001 a | ||||||||
| Urban | 134 (43.8) | 10 (97.5) | 0 (0.0) | 24 (17.9) | 37 (27.6) | 34 (25.4) | 24 (17.9) | 5 (3.7) | |
| Rural | 171 (55.9) | 5 (2.9) | 4 (2.3) | 9 (5.3) | 34 (19.9) | 105 (61.4) | 13 (7.6) | 1 (0.6) | |
| Nomadic | 1 (0.3) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Occupation | < 0.001 a | ||||||||
| Housewife | 142 (46.4) | 3 (2.1) | 1 (0.7) | 18 (12.7) | 35 (24.6) | 55 (38.7) | 25 (17.6) | 5 (3.5) | |
| Student | 10 (3.2) | 2 (20.0) | 1 (10.0) | 1 (10.0) | 2 (20.0) | 4 (40.0) | 0 (0.0) | 0 (0.0) | |
| Worker | 48 (15.7) | 3 (6.3) | 1 (2.1) | 1 (2.1) | 12 (25.0) | 27 (56.3) | 4 (8.3) | 0 (0.0) | |
| Free | 54 (17.6) | 2 (3.7) | 0 (0.0) | 9 (16.7) | 6 (11.1) | 34 (63.0) | 3 (5.6) | 0 (0.0) | |
| Prisoner | 2 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (50.0) | 1 (50.0) | 0 (90.0) | 0 (0.0) | |
| Unemployed | 17 (5.6) | 0 (0.0) | 0 (0.0) | 1 (5.9) | 7 (41.2) | 8 (47.1) | 1 (5.9) | 0 (0.0) | |
| Disabled | 12 (4.0) | 4 (33.3) | 0 (0.0) | 0 (0.0) | 4 (33.3) | 2 (16.7) | 2 (16.7) | 0 (0.0) | |
| Others | 21 (6.9) | 1 (4.8) | 1 (4.8) | 4 (19.0) | 4 (19.0) | 8 (38.1) | 2 (9.5) | 1 (4.8) | |
a There is statistical significance based on the year of the disease
Out of 128 cases of HCV, 85.1% were men, with a mean age of 45.12 ± 13.31 years; the age groups of 50 years or older and 30-39 years with 33.6 and 29.7% had the highest frequency, respectively. It is important to note that no cases of HCV were identified in the age category of 0-9 years during the mentioned period. Most cases were of Iranian nationality, and 79.7% lived in urban areas. Except for marital status, which demonstrated statistically significant patterns during different years (P = 0.005), other variables were not significantly different by year and disease (P > 0.05) (Table 2).
| Variables | Total | Year of Occurrence | P-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | |||
| Age, years | 0.810 a | ||||||||
| 0 - 9 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 10 - 19 | 1 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 20 - 29 | 11 (8.6) | 1 (9.1) | 0 (0.0) | 1 (9.1) | 4 (36.4) | 4 (36.4) | 0 (0.0) | 0 (0.0) | |
| 30 - 39 | 38 (29.7) | 0 (0.0) | 1 (2.6) | 2 (5.3) | 16 (42.1) | 11 (28.9) | 1 (2.6) | 1 (2.6) | |
| 40 - 49 | 35 (27.3) | 3 (8.6) | 0 (0.0) | 4 (11.4) | 15 (42.9) | 11 (31.4) | 0 (0.0) | 0 (0.0) | |
| 50 ≤ | 43 (33.6) | 1 (2.3) | 0 (0.0) | 8 (18.6) | 13 (30.2) | 15 (34.9) | 0 (0.0) | 0 (0.0) | |
| Gender | 0.166 a | ||||||||
| Male | 109 (85.1) | 4 (3.7) | 1 (0.9) | 9 (8.3) | 43 (39.4) | 37 (33.9) | 14 (12.8) | 1 (0.9) | |
| Female | 19 (14.9) | 1 (5.3) | 0 (0.0) | 6 (31.6) | 6 (31.6) | 4 (21.1) | 2 (10.5) | 0 (0.0) | |
| Nationality | 0.966 a | ||||||||
| Iranian | 122 (95.3) | 5 (4.1) | 1 (0.8) | 15 (12.3) | 46 (37.7) | 39 (32.0) | 15 (12.3) | 1 (0.8) | |
| Afghan | 6 (4.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (50.0) | 2 (33.3) | 1 (16.7) | 0 (0.0) | |
| Marital Status | 0.005 b | ||||||||
| Single | 22 (17.1) | 1 (4.5) | 0 (0.0) | 1 (4.5) | 15 (68.2) | 1 (4.5) | 3 (13.6) | 1 (4.5) | |
| Married | 106 (82.9) | 4 (3.8) | 1 (0.9) | 14 (13.2) | 34 (32.1) | 40 (37.7) | 13 (12.3) | 0 (0.0) | |
| Place | 0.101 a | ||||||||
| Urban | 102 (79.7) | 4 (3.9) | 0 (0.0) | 13 (12.7) | 34 (33.3) | 35 (34.3) | 15 (14.7) | 1 (1.0) | |
| Rural | 26 (20.3) | 1 (3.8) | 1 (3.8) | 2 (7.7) | 15 (57.7) | 6 (23.1) | 1 (3.8) | 0 (0.0) | |
| Nomadic | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
a It is not statistically significant based on the year of occurrence of the disease.
b There is statistical significance based on the year of the disease.
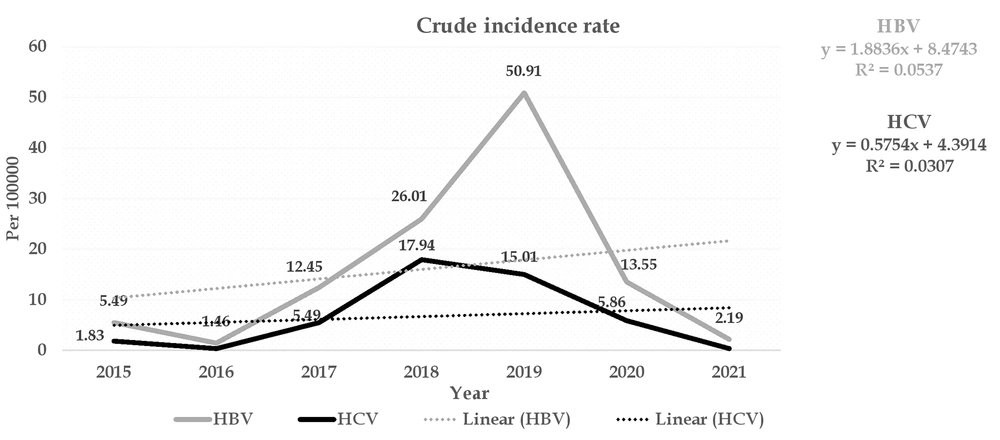
The overall incidence of HBV and HCV was estimated at 18.44 and 7.71 per 100,000 population, respectively. The highest incidence of HBV was observed at 50.91 and 26.01 per 100,000 population in 2019 and 2018, respectively, and the lowest incidence was reported in 2016 (1.46 cases per 100,000 population). The highest incidence of HCV, with 17.94 and 15.01 per 100,000 population, was reported in 2018 and 2019, respectively, and the lowest incidence (0.36 per 100,000 population) was in 2016. The finding of the Cochrane-Armitage trend test for both types of HBV and HCV showed that the incidence of hepatitis from 2015 to 2021 was statistically significant and had an increasing trend (P Trend < 0.001) (Figure 1).
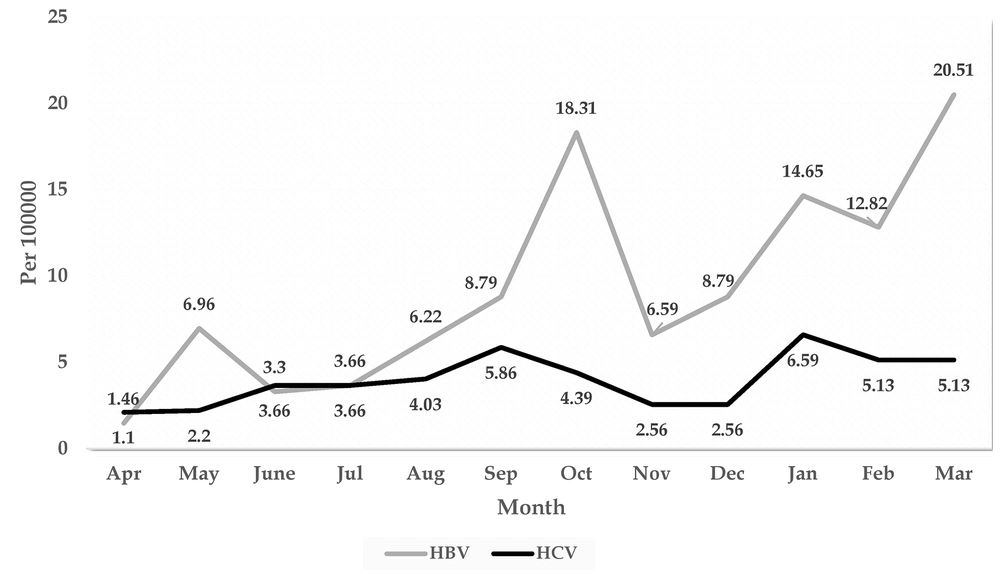
The results displayed in Figure 2 demonstrate that the highest incidences of HBV, with 20.51, 18.31, 14.65, and 12.82 per 100,000 population, were observed in March, October, January, and February, respectively. The highest incidence of HCV was reported in January (6.59 per 100,000 population) and September (5.86 per 100,000 population), respectively. The lowest incidence for both hepatitis cases was observed in April (Figure 2).
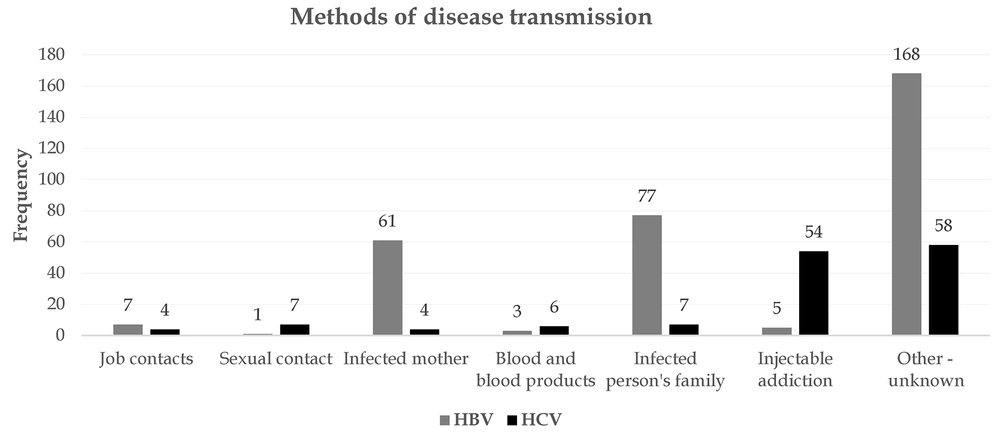
The results of HBV transmission routes showed that the infection of one of the family members and an infected mother were the most important causes of the disease. Seven subjects became infected through accidental occupational contacts, and 168 patients were infected by other methods such as surgery and dental procedures. On the other hand, injecting drug use was the most common cause of HCV infection. Extramarital sexual contact, concomitant infection of family members, and transfusion of blood and blood products were the next most important causes. (Figure 3).
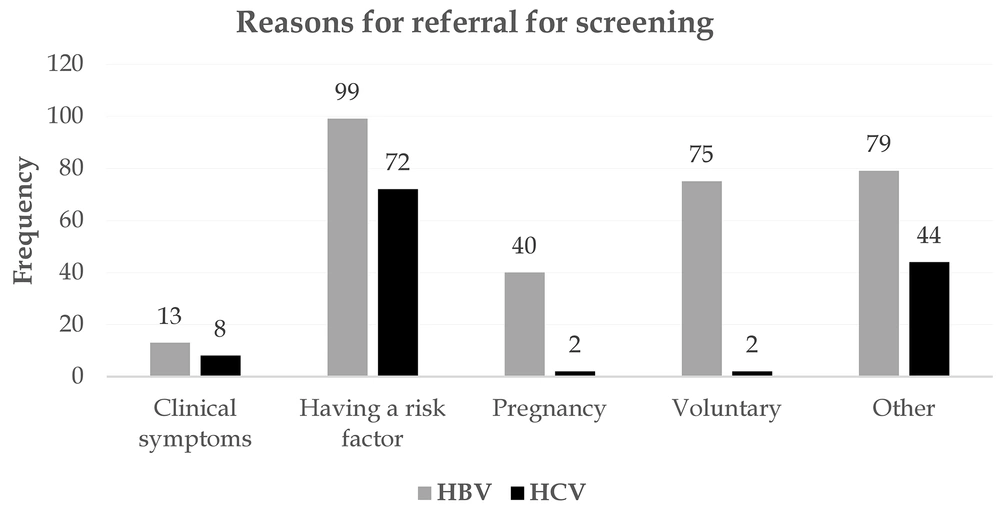
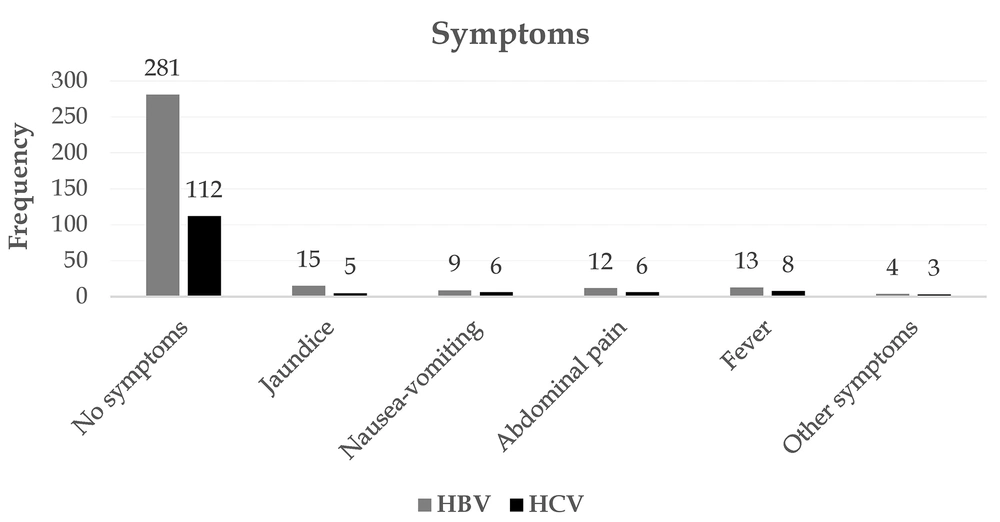
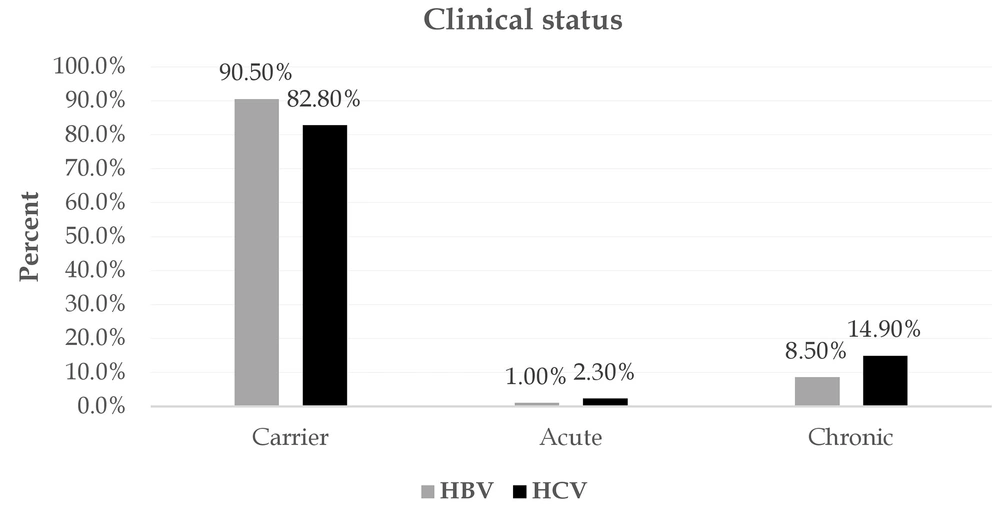
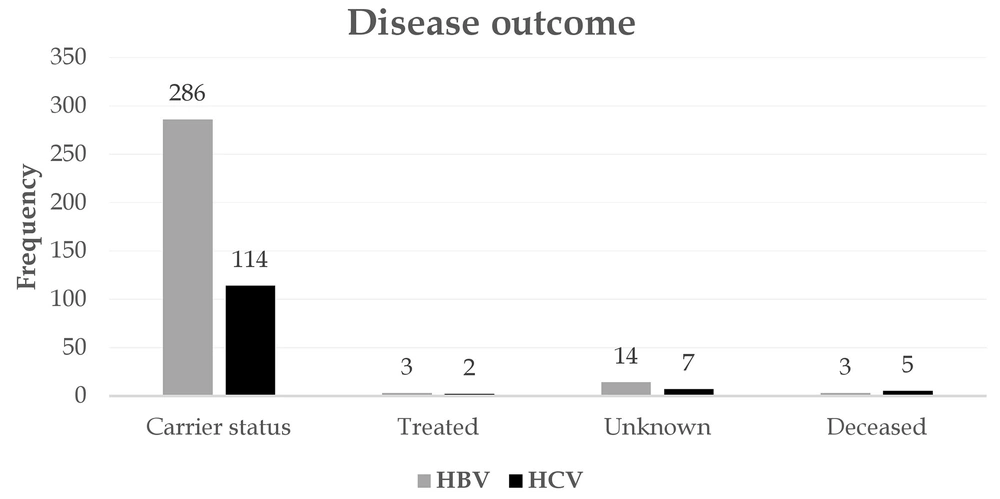
Figure 4 depicts the most important reasons for referral for screening. A potential risk factor, voluntary referral, and pregnancy were the most important reasons for referral for screening of patients with HBV. Seventy-two subjects were also tested for a risk factor and were eventually confirmed to have HCV. In both HBV and HCV, most patients showed no clinical presentations. Jaundice, abdominal pain, and fever were the most common symptoms associated with the disease (Figure 5). Among patients with HBV and HCV, 90.5% and 82.80%, respectively, were carriers, and only a very small proportion experienced acute conditions (Figure 6). Figure 7 shows the disease outcome; three and five subjects with HBV and HCV, respectively, died, and most patients are currently carriers.
5. Discussion
According to the findings of this study, most subjects were infected with HBV in the age groups of 50 and above and 20 - 29 years. The mean age of the participants was approximately 40 years. Also, most subjects with HCV were in the age groups of 50 and above (33.6%) and 30 - 39 years (29.7%), with a mean age of about 45 years. In a study carried out by Hyun Kim and Ray Kim in the United States on the epidemiology of HBV infection, the findings showed that between 2000 and 2012, there was a declining trend in the prevalence of the disease in all age groups. The age group that showed the greatest decrease in HBV incidence were children and those in their 20s. Some patients had received the HBV vaccine in infancy. In contrast, in other age groups (30 to 49 years), HBV incidence was rising. In the study by Hyun Kim and Ray Kim, one of the reasons for the recurrence of this disease in the age group of 30 to 49 years was mentioned as drug injection by this age group (18). In a study by Rezaee-zavareh and Alavian, the prevalence of HCV in the population aged 35 to 44 years was 38.5%, and in those aged 25 - 34 years, it was 26.9% (19). The effect of drug injection on HBV infection was also determined in a study by Shing et al. (20). One-fifth of adults with a history of intravenous drug injection had HBV infection, four times more common than in the general population (20). In the present study, injecting drugs was one of the important methods of infection transmission.
In the present study, most subjects with HBV were female (53.6), while in the study conducted by Al-Busafi et al. in Oman on the risk factors for HBV infection, most subjects were male (52.2%). Piercing in non-clinical settings was one of the women's most important causes of infection transmission (21). A comparison of the present study with Al-Busafi did not show any significant sex differences in subjects with HBV. Regarding the incidence of HCV, most subjects (85.1%) were male. In a study by Shahriari-Fard et al., it was reported that men were more than four times more likely to contract HCV. The researchers in this study argued that this might be due to a higher rate of IV substance abuse by men (22).
In the present study, 45.1% of HBV subjects were from Afghanistan. Sievert et al. (23) reported language and cultural barriers, as well as lack of HBV knowledge, as the most important factors in the lack of access to chronic HBV testing and treatment in the Afghan population, which emphasizes the importance of awareness raising and educating this high-risk group.
In the present study, most subjects with HBV and HCV were married. In the study of Kpossou et al., 50% of the subjects with HBV were married (24). Therefore, it can be concluded that marriage is not a protective factor against HBV and HCV infection. Also, most studied individuals were from rural areas, which may be due to the low level of information about HBV and HCV transmission methods in these people. In a study conducted by Adam and Fusheini in Ghana, one of the most important reasons for HBV infection in rural areas was the lack of knowledge about this disease (25).
The trend of HBV and HCV in the current research revealed that despite the sudden increase in the incidence of HBV and HCV in 2018 and 2019, the rate of HBV and HCV in 2020 and 2021 decreased. Generally, between 2015 and 2021, there was a fluctuating trend in the prevalence of HBV and HCV. In a study by Sajjadi et al. in Kohgiluyeh and Boyer-Ahmad, Iran, researchers also showed a fluctuating trend in the incidence of HBV and HCV infections between 2005 and 2014. The researchers in this study attempted to increase the general knowledge about infections that could be transmitted through blood transfusions and the risk factors and ways of transmitting these diseases. They also cited improving public health programs as one of the reasons for successfully reducing these infections (26).
In the current study, the finding of the Cochrane-Armitage trend test for both HBV and HCV showed that the incidence of hepatitis from 2015 to 2021 had significantly increased. One of the reasons for the high rate of viral hepatitis in the studied population can be the immigration in the study area (especially Afghan immigrants). The findings demonstrated that the number of Afghan immigrants with HBV and HCV is high. According to the World Health Organization, viral hepatitis, such as HBV and HCV, are considered a health concern in Afghanistan. Moreover, the World Health Organization has emphasized the need for education and vaccination against hepatitis to reduce the number of hepatitis cases in Afghanistan (27, 28). In other studies, on the effect of geographical location, mass migration from countries like Afghanistan, and illegal drug traffic from eastern borders with Pakistan and Afghanistan, the spread of viral hepatitis in Iran has been emphasized (29, 30).
In the present study, the most important causes of HBV and HCV were transmission through other family members, transmission through pregnancy, injection of drugs, unsafe sexual contact, and transmission through infected blood. Evidence shows that transmission between family members in such infections might occur in communities with poor socio-economic status and health (31). In 2019, researchers in a study conducted in Africa also reported that mothers infected with HBV or HCV had a high potential for transmitting the infection to their children (32). In a study in Pakistan, the most important causes of transmission of HBV and HCV infection were reported to be through drug injection, contaminated blood, and in the workplace among healthcare workers (33). Consistent with the present study, various studies have stated that unsafe sexual contact is one of the main routes of HBV and HCV transmission (34, 35). In the present study, many patients were diagnosed with HBV and HCV during prenatal testing. Hepatitis screening is not necessary for Iranian pregnant women. However, pregnant women with a history of high-risk behaviors are referred for HIV and HBsAg testing to receive care (36). Therefore, many of these infections are detected during such prenatal tests. Meanwhile, some researchers have recommended screening for HBV and HCV surface antigens in pregnant women in Iran (37, 38).
In the present study, most samples had no clinical presentations. In this case, too, researchers state that many cases of B and C infections remain asymptomatic and are usually diagnosed accidentally during pregnancy, blood donation, screening, or because of a positive case in other family members (39). However, jaundice, abdominal pain, and fever were the most important symptoms identified in these patients in the present study. These clinical presentations are reported in other studies, too (40, 41). Most subjects were HBV and HCV carriers. Evidence shows that the number of HBV and HCV carriers worldwide is approximately 350 million (42). Therefore, screening tests, when necessary, such as blood donation, can reduce the prevalence of these infections in communities (26). Most deaths from viral hepatitis are due to HBV and HCV. HBV and HCV are also one of the most important reasons for the risk of contracting a dangerous group of fatal diseases, including hepatocellular carcinoma (HCC) and liver cirrhosis (43). The mortality rate in the samples of the present study was low, and most were carriers. In the study of Kumada et al. in Japan, the mortality rate of carriers of HBV infection was not significantly different from that of the general population (44).
5.1. Limitations
In the present study, only the researchers had access to the registered files, and the information of some people may not have been registered. Moreover, the researchers did not know the total number of screened people and only had access to the information of people with positive screening tests.
5.2. Conclusions
In general, the incidence of HBV and HCV has increased significantly. It was demonstrated that important epidemiological indicators related to HBV and HCV in this study were very different from other studies conducted in Iran. Thus, it is crucial to prioritize preventive and control initiatives in this particular region with greater care than in other regions. Educating people in the community, especially high-risk groups such as IV drug users and those with unsafe sex, can greatly reduce the incidence of the disease. On the other hand, daily monitoring with high sensitivity in detecting infection in blood transfusion centers can reduce virus transmission to certain patients, including dialysis, hemophilia, and thalassemia patients. It is important to note that, given the routine HBV vaccination schedule in infants, we are likely to see changes in the epidemiological pattern of the disease in this area over the coming years, and adults will be at higher risk for the disease.