1. Background
Hypertension (HTN), or high blood pressure (BP), is defined as when systolic and diastolic blood pressures are higher than the normal range, namely 140 and 90, respectively. Hypertension is a global public health problem with an increasing trend in low-income and middle-income countries than in high-income countries (1). It is part of a clinical disease that is drawn from complicated etiologies and leads to the development of complex cardiovascular disease (CVD), including coronary artery diseases, angina, and myocardial infarction (2). Based on the WHO report, the prevalence of HTN is between 14.7% and 26.4% in different countries of the Eastern Mediterranean region (3). The prevalence of HTN in Iran is reported to be 19.2% and is relatively higher in men than women (4). Therefore, the management of hypertension is clinically imperative and can lead to significant public health benefits (5).
According to American Heart Association guidelines, patients with HTN should follow certain dietary modifications (6). Long-standing evidence shows that consumption of specific food groups, including whole grains, fruit, nuts, legumes, and dairy products, is associated with a significant decrease in hypertension risk (7).
Previous studies reported that adherence to the dietary approach to stop hypertension (DASH) diet was associated with a lower risk of hypertension. Dietary adherence is a key health behavior to promote blood pressure control among individuals with hypertension (8-10).
Dietary approaches to stop hypertension is an eating pattern that emphasizes the consumption of fruits, vegetables, whole grains, and low-fat dairy products. It also contains small amounts of dietary sodium, saturated fat, meat, and sweetened beverages. Dietary approach to stop hypertension is one of the most generally recommended dietary modifications for reducing BP and CVD risk (6) and has been suggested as a first-line nonpharmacological for the treatment of many chronic diseases (11).
Hence, medical treatment alone is inadequate for blood pressure control, and other interventions are needed to change behavior that has broad potential to alter current patterns of the disease (12). Self-efficacy is defined as an individual’s sureness in their ability and capability to successfully plan and participate through a series of actions that will result in anticipated outcomes. Previous studies showed the fundamental role of self-efficacy in a better self-caring process for chronic disease (13-15).
The finding of a recent systematic review and meta-analysis of fourteen studies concluded that self-management education programs led to a statistically significant increase in the self-efficacy of the participants as well as a significant decrease in systolic blood pressure (SBP) and diastolic blood pressure (DBP) compared to control groups (16). In fact, people with higher self-efficacy tend to set higher goals, anticipate greater outcomes, and persevere in the challenges without surrendering.
Mobile health apps have become increasingly popular in delivering health messages to patients. Nowadays, more than 100,000 of these health-related apps are used by patients with different diseases (17), and many have been developed for people with hypertension. In fact, these apps recommend different actions for self-management of the disease through several activities such as reminders, evidence-based information, and feedback (18, 19).
2. Objectives
The current study aimed to investigate the efficiency of mobile apps in improving self-efficacy in adherence to the DASH diet in HTN patients.
3. Methods
3.1. Design
The present study was a parallel-group randomized controlled trial to evaluate the effectiveness of mobile phone education based on self-efficacy and DASH diet among patients with high blood pressure.
3.2. Participants
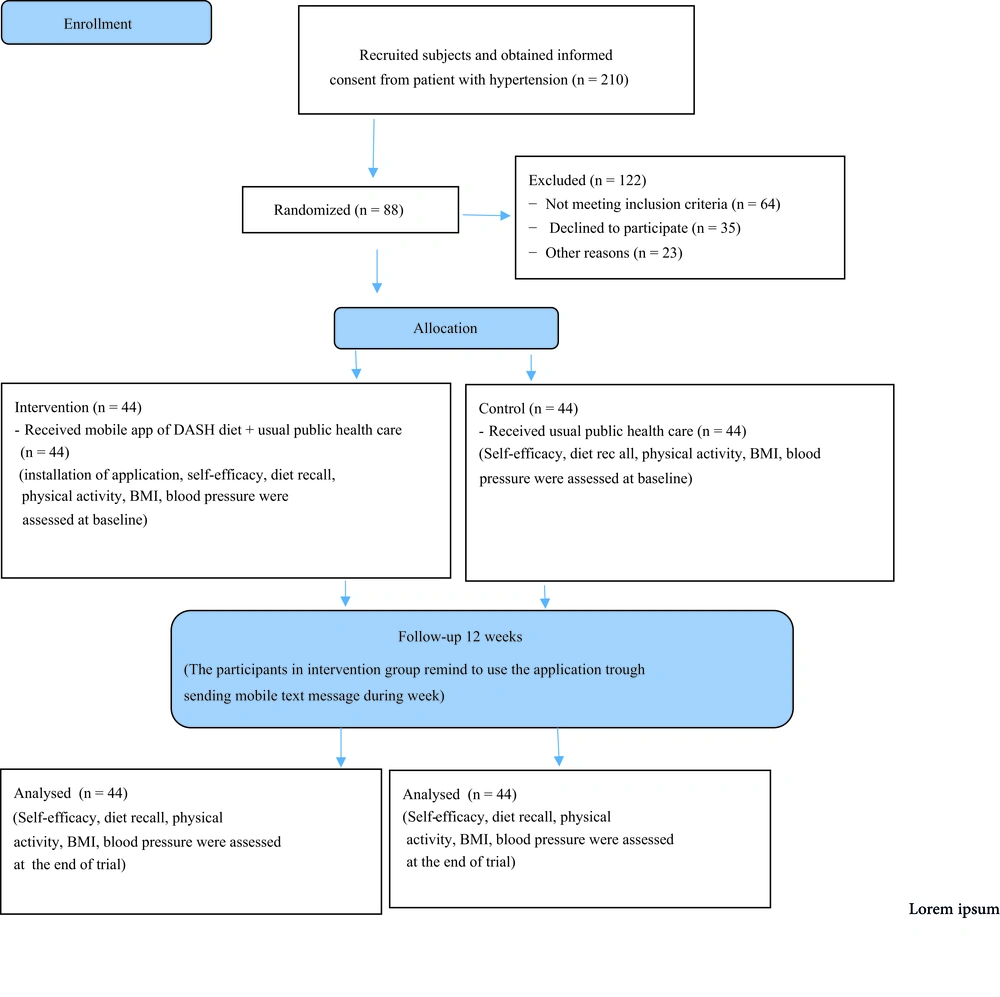
A total of 210 patients with hypertension were provided informed consent, and 110 patients refused to participate; 64 did not meet the inclusion criteria, 35 declined to attend the study, and 11 were excluded for other reasons that were not mentioned exactly. Hence, 100 patients were interested in participating in the study (Figure 1).
All participants aged between 30 and 69 years with established high blood pressure according to available guidelines (6). This study is reported in line with CONSORT guidelines. Inclusion criteria were as follows: The patient had the desire to participate, aged more than 25 years old, had a disease history of at least 12 months before the day of inclusion, BMI > 25, being overweight or obese, and had blood pressure was 130 to 139 or higher for systolic and 80 to 89 or higher for diastolic, taking anti-hypertensive drug(s) for at least 6 months, having a smartphone, having phone literacy, and residence of Ahvaz city. Subjects were not included in the present study if they had a history of renal, arrhythmia, gastrointestinal, hepatic, endocrinological, or hematological disease. In addition, patients were also excluded from participating in another study simultaneously, following a specific diet regime without the intention of continuing the study, changing the doses of anti-hypertensive drugs, and treatment status.
3.3. Study Setting and Procedure
The patients who were referred to the public health care center to receive the usual health care program were recruited to the present study between November 2019 and March 2020. Hence, after stating their desire to attend the study, they were informed of the objectives of the study. Therefore, interested patients were randomly assigned to either the intervention or the control group. The present study was a randomized controlled clinical trial. All subjects from urban public health care centers were randomly allocated into two different groups by 8 strata for age (30 - 39, 40 - 49, 50 - 59, 60 - 69) and gender (male and female). Randomization was obtained using computer-generated random numbers by a computer assistant.
3.4. Intervention
The intervention group received the mobile app for DASH-related recommendations. This group also received usual health-related recommendation texts. The participants were strictly advised to follow all recommendations and adjust their nutritional habits. Compliance with the intervention was controlled by the nutritionist through weekly phone interviews and short text messages. The control group received usual health-related recommendations in a public health care center.
The smartphone app was an Android-based offline application that contained DASH diet recommendations on the basis of self-efficacy. It also included two separate realistic short videos that were considered in the application: One from a patient with hypertension who followed the DASH diet and the concluding remarks of a nutrition expert emphasizing the importance of nutrition in controlling HTN. In addition, in another video, the importance of the DASH diet in controlling hypertension was stated from the point of view of a specialist in dietetics. The concept of the program was designed to improve the self-efficacy of participants. All recommendations in this application were based on accepted guidelines in this context. In addition, an integrated message was sent to all participants by phone call and text messages as a reminder at specific time points. At the end of the study, the control group also recommended adopting education materials for implementation.
3.5. Measurements
Body weight was measured in light clothes without shoes by the use of a digital scale (Seca, Hamburg, Germany) to the nearest 0.1 kg. Height was calculated to the nearest 0.1 cm by using an inelastic tape measure (Seca, Hamburg, Germany). Body mass index was also calculated according to the established formula: Weight (kg)/height2 (m). Physical activity levels were evaluated at the beginning and the end of the intervention through the Iranian International Physical Activity Questionnaire (IPAQ). Therefore, the physical activity level of all participants was reported as the metabolic equivalent of task (MET) minutes/week.
All patients completed a general questionnaire comprising different variables (including age and sex) and lifestyle habits (including the history of smoking and alcohol consumption). Medical and drug history, family history of diseases, and hypertension duration were also recorded. Demographic background information of the participants was obtained according to age (through open-ended questions), education level (< 12 grade vs. > 12 grade), and family income status (good, moderate, poor).
Dietary intake was measured using an average diet recall for three days (including 2 working days and 1 weekend day) a week before and at the end of the intervention. The dietary record was analyzed using Nutritionist IV software (First Databank, San Bruno, CA, USA) and was adjusted for Iranian foods (20).
The score for DASH diet adherence was computed according to nine target nutrients, specifically total fat, saturated fat, protein (indexed to total energy intake), fiber, cholesterol, calcium, magnesium, sodium, and potassium. Then, micronutrients were expressed per 1000 kcal. The DASH score was made by the sum of all nutrient target values (maximum 9): A value of 1 was considered for participant who met the DASH target for a nutrient, a value of 0.5 was given if met the intermediated target, and the value of zero was assigned if neither target was met. Dietary approach to stop hypertension adherence was considered by a total score of ≥ 4.5 out of 9 (21).
Hypertension was defined as blood pressure ≥ 130/85 mmHg based on reliable guidelines (6). Systolic blood pressure and DBP were measured twice by health staff after a 10-minute rest using a citizen digital blood pressure monitor. The average of the two measurements was used for analysis (22). The self-efficacy questionnaire in weight management was first introduced by Clark et al. (23).
This questionnaire evaluates the different beliefs of participants about their life events and contains 30 different questions in which, based on Likert scoring, they could score between 0 to 4. The minimum and maximum scores could be between 0 and 120. Higher scores demonstrate higher self-efficacy in participants to their abilities, and the estimated alpha Cronbach is reported to be between 0.7 to 0.9. The self-efficacy questionnaire was validated for the Iranian population by Navidian et al. (24), and they reported appropriate validity and reliability for the Iranian population.
3.6. Sample Size
The sample size was calculated according to type one (a) and type two errors (b) as 0.05 and 0.20 (power = 80%), respectively. Based on a previously published study, the standard deviation (SD) and mean difference in diastolic blood pressure were 82.52 ± 3.28 and 84.84 ± 3.69 for the DASH diet and control group, respectively (25). The sample size was determined based on comparing the mean of two groups of treatment. Because of a possible 20% reduction, a total of 44 participants were anticipated for each arm of the study.
3.7. Randomization Type
Based on the simple computerized randomization, participants were allocated either to the intervention or the control group.
3.8. Allocation Concealment
The educational intervention applied for participants was based on the randomization schedule odd/ even number order.
3.9. Statistical Analysis
The normal distribution of all variables was tested by the Kolmogorov-Smirnov test. All measured variables were expressed as mean and SD for quantitative variables or number and percentage for qualitative variables. To compare continuous variables with normal and abnormal statistical distribution, the paired-sample t-test and Wilcoxon test were used to compare the results within groups at the end of the trial, respectively. An independent sample t-test with normal and Mann-Whitney test with abnormal statistical distribution was performed to compare the results between the two groups at the end of the trial. Analysis of covariance was performed to identify any differences between the two groups at the end of the study, adjusting for baseline values (macronutrients, sodium, age, BMI, physical activity).
The Mellen et al. formula was utilized for the calculation of adherence to the DASH diet (21). Besides, the 9 nutrients inclusive of saturated fatty acid (g/d), total fat (g/d), total protein (g/d), cholesterol (g/d), fiber (g/d), magnesium (mg/d), calcium (mg/d), potassium (mg/d) and sodium (mg/d) were considered for calculation of the DASH score. Moreover, a maximum 9 score was considered by summation of all nutrients that achieved the target level score, and the intermediate target level was considered ≥ 4.5, and lower than the intermediate levels did not meet the DASH target level. Furthermore, some nutrients were considered as a proportion of the total daily energy consumption, and the rest of the nutrients accounted per 1000 kcal of energy intake. They are all elaborated in Appendix 1.
Statistical significance was determined at a P-value of less than 0.05. All statistical analyses were carried out using the statistical package for social science version 17 (SPSS Inc., Chicago, Illinois, USA).
3.10. Ethics
All procedures were in accordance with the Declaration of Helsinki, and the subjects provided written informed consent. The study protocol was approved by the Ahvaz Jundishapur University of Medical Sciences Ethical Committee (IR.AJUMS.REC.1398.419). The study is registered under the Iranian Registry of Clinical Trials with the IRCT number: IRCT20190930044933N1.
4. Results
Among 100 patients allocated to the study groups, 88 patients were included in the final analysis. The reason for dropping out is shown in Figure 1.
At the beginning of the study, the demographic characteristics did not significantly differ between the two groups. Food recall information also showed that at the beginning of the study, there were no significant differences between the two groups in dietary intake of macronutrients, total energy, carbohydrates, proteins, fats, and only intake of fibers were significantly different (Table 1). The independent sample t-test showed there were no significant differences in DBP and SBP at the baseline, but intervention led to a significant decrease in both SBP and DBP. Body mass index was not significantly different at the baseline and end of the study between study groups. However, there was a significant difference in the intervention group at the end of the study (Table 2).
| Macro and Micronutrient Characteristics of Participants | Intervention Group (n = 44) | Control Group (n = 44) | P-Value |
|---|---|---|---|
| Calories (kcal/d) | |||
| Baseline | 2628.55 ± 437.36 | 2806.33 ± 874.62 | 0.3 |
| End of trial | 2387.34 ± 409.61 | 2618.96 ± 362.45 | 0.006 |
| P | 0.0001 | 0.5 | |
| Protein (g/d) | |||
| Baseline | 83.29 ± 16.04 | 77.80 ± 14.64 | 0.06 |
| End of trial | 88.14 ± 14.71 | 79.75 ± 15.67 | 0.01 |
| P | 0.05 | 0.0001 | |
| Fiber (g/1000 kcal) | |||
| Baseline | 12.40 ± 4.47 | 16.61 ± 3.35 | 0.0001 |
| End of trial | 14.93 ± 4.97 | 16.21 ± 0.51 | 0.07 |
| P | 0.001 | 0.3 | |
| Fat (g/d) | |||
| Baseline | 66.46 ± 20.40 | 66.99 ± 21.39 | 0.9 |
| End of trial | 56.87 ± 20.56 | 61.99 ± 17.3 | 0.03 |
| P | 0.0001 | 0.0009 | |
| Carbohydrate (g/d) | |||
| Baseline | 215.57 ± 36.61 | 201.43 ± 42.13 | 0.09 |
| End of trial | 192.25 ± 40.12 | 192.51 ± 40.30 | 0.9 |
| P | 0.0001 | 0.03 | |
| MUFA (g/d) | |||
| Baseline | 29.34 ± 8.64 | 31.29 ± 10.56 | 0.2 |
| End of trial | 24.27 ± 9.40 | 28.37 ± 8.33 | 0.4 |
| P | 0.0001 | 0.02 | |
| PUFA (g/d) | |||
| Baseline | 13.09 ± 3.64 | 14.73 ± 4.26 | 0.03 |
| End of trial | 12.09 ± 3.86 | 15.16 ± 4.09 | 0.001 |
| P | 0.1 | 0.5 | |
| Cholesterol (mg/d) | |||
| Baseline | 480.45 ± 146.85 | 442.99 ± 153.52 | 0.9 |
| End of trial | 389.09 ± 140.72 | 454.15 ± 136.68 | 0.8 |
| P | 0.0001 | 0.1 | |
| Saturated fat (g/d) | |||
| Baseline | 13.44 ± 2.31 | 15.43 ± 2.33 | 0.0001 |
| End of trial | 13.28 ± 2.32 | 15.25 ± 3.10 | 0.001 |
| P | 0.5 | 0.2 | |
| Potassium (mg/d) | |||
| Baseline | 996.69 ± 238.001 | 819.43 ± 358.51 | 0.0001 |
| End of trial | 1285.31 ± 396.98 | 781.03 ± 394.01 | 0.0001 |
| P | 0.0001 | 0.08 | |
| Zn (mg/d) | |||
| Baseline | 7.93 ± 0.54 | 7.88 ± 0.54 | 0.5 |
| End of trial | 7.83 ± 0.56 | 7.90 ± 0.57 | 0.4 |
| P | 0.3 | 0.9 | |
| Calcium (mg/d) | |||
| Baseline | 193.34 ± 88.13 | 186.92 ± 74.61 | 0.8 |
| End of trial | 209.51 ± 90.31 | 186.87 ± 74.95 | 0.2 |
| P | 0.0001 | 0.4 | |
| Magnesium (mg/d) | |||
| Baseline | 148.07 ± 62.53 | 143.60 ± 61.12 | 0.7 |
| End of trial | 174.25 ± 73.17 | 130.85 ± 55.71 | 0.003 |
| P | 0.0001 | 0.02 | |
| Selenium (µg/d) | |||
| Baseline | 0.3817 ± 0.13 | 0.3910 ± 0.13 | 0.7 |
| End of trial | 0.39 ± 0.14 | 0.40 ± 0.13 | 0.9 |
| P | 0.2 | 0.2 | |
| Vitamin C (mg/d) | |||
| Baseline | 89.80 ± 17.91 | 89.70 ± 17.98 | 0.9 |
| End of trial | 98.55 ± 16.26 | 99.88 ± 14.92 | 0.8 |
| P | 0.003 | 0.001 | |
| Vitamin E (mg/d) | |||
| Baseline | 3.87 ± 0.54 | 4.03 ± 0.50 | 0.1 |
| End of trial | 4.07 ± 0.53 | 4.19 ± 0.42 | 0.3 |
| P | 0.1 | 0.09 | |
| Vitamin A (µg/d) | |||
| Baseline | 825.71 ± 72.05 | 881.68 ± 74.95 | 0.0001 |
| End of trial | 861.93 ± 100.004 | 879.55 ± 98.84 | 0.5 |
| P | 0.03 | 0.9 |
a Values are expressed as mean ± SD.
| Patient Characteristics and Macronutrients | Intervention Group (n = 44) | Control Group (n = 44) | P-Value |
|---|---|---|---|
| Number of patients (female) (%) | 48.9 | 51.1 | 0.6 |
| Age (y) | 49.45 ± 10.1 | 49.42 ± 9.63 | 0.9 |
| Income status (%) | 0.9 | ||
| Good | 69.2 | 30.8 | |
| Moderate | 50.9 | 49.1 | |
| Poor | 33.3 | 66.7 | |
| Education status (%) | 0.3 | ||
| ≤ 12 grade | 62.5% | 37.5% | |
| > 12 grade | 48.8% | 51.2% | |
| Physical activity (met min/week) | 1989.96 ± 1163.83 | 1780.40 ± 1036.93 | 0.2 |
| BMI (kg/m2) | |||
| Baseline | 29.51 ± 2.89 | 28.53 ± 2.57 | 0.1 |
| End of trial | 29.40 ± 2.91 | 28.64 ± 2.62 | 0.2 |
| P | 0.002 | 0.05 | |
| HTN duration, (n) | 0.5 | ||
| Less than 5 year | 30 | 28 | |
| More than 5 year | 14 | 16 |
a Values are expressed as mean ± standard deviation unless otherwise indicated.
Results from self-efficacy showed that baseline values of the three components were not significantly different between the two groups. However, the result of ANCOVA demonstrated that all five components improved significantly at the end of the trial (Table 3). At the end of the study, there were no significant statistical differences regarding adherence to the DASH diet within study groups, as tabulated in Table 3.
| Factors Components | Intervention Group | Control Group | P-Value c |
|---|---|---|---|
| F1 | |||
| Baseline | 12.05 ± 8.33 | 14.2 ± 6.33 | 0.2 |
| End of trial | 21.72 ± 2.9 | 9.26 ± 6.65 | 0.001 |
| P | 0.0001 d | 0.0001 d | |
| M1 e | 21.78 (2.22, 23.33) | 9.21 (7.66, 10.76) | 0.0001 |
| M 2 e | 21.90 (19.94, 23.86) | 9.35 (7.39, 11.31) | 0.0001 |
| F2 | |||
| Baseline | 2.38 ± 1.68 | 2.83 ± 2.4 | 0.4 |
| End of trial | 15.52 ± 4.63 | 5.16 ± 4.37 | 0.00001 |
| P | 0.0001 | 0.0001 | |
| M1 | 8.90 (7.68, 10.12) | 3.99 (2.78, 5.21) | 0.0001 |
| M 2 | 9.0 (7.97, 10.02) | 3.89 (2.86, 4.93) | 0.0001 |
| F3 | |||
| Baseline | 5.75 ± 3.55 | 7.11 ± 2.39 | 0.0001 |
| End of trial | 8.26 ± 2.72 | 3.93 ± 3.05 | 0.0001 |
| P | 0.0001 | 0.004 | |
| M1 | 11.96 (10.5, 13.43) | 7.89 (6.43, 9.36) | 0.001 |
| M 2 | 12.64 (11.32, 13.95) | 7.22 (5.90, 8.53) | 0.00001 |
| F4 | |||
| Baseline | 1.22 ± 1.23 | 1.64 ± 2.03 | 0.0001 |
| End of trial | 0.9 ± 1.45 | 0.81 ± 4.43 | 0.1 |
| P | 0.05 | 0.004 | |
| M1 | 5.99 (5.12, 6.85) | 3.88 (3.02, 4.75) | 0.001 |
| M 2 | 5.79 (4.76, 6.81) | 4.09 (3.06, 5.11) | 0.04 |
| F5 | |||
| Baseline | 0.086 ± 3.08 | 1.23 ± 2.56 | 0.1 |
| End of trial | 0.87 ± 1.58 | 0.41 ± 1.98 | 0.4 |
| P | 0.08 | 0.2 | |
| M1 | 2.89 (2.6, 3.16) | 1.26 (1.0, 1.53) | 0.0001 |
| M | 2.86 (2.55, 3.17) | 1.29 (0.98, 1.60) | 0.0001 |
| BP. systolic (mmHg) | |||
| Baseline | 150.43 ± 10.19 | 155.88 ± 16.81 | 0.06 |
| End of trial | 144.65 ± 10.36 | 161.09 ± 17.46 | 0.0001 |
| P | 0.0001 | 0.0001 | |
| M1 | 144.76 (140.51, 149.007) | 160.98 (156.74, 165.23) | 0.0001 |
| M 2 | 144.28 (137.29, 151.28) | 161.46 (154.46, 168.45) | 0.0001 |
| BP. Diastolic (mmHg) | |||
| Baseline | 94.15 ± 7.69 | 96.13 ± 8.41 | 0.7 |
| End of trial | 88.59 ± 8.34 | 97.61 ± 7.27 | 0.0001 |
| P | 0.0001 | 0.1 | |
| M1 | 88.49 (86.22, 90.75) | 97.71 (95.44, 99.97) | 0.0001 |
| M 2 | 85.89 (82.33, 89.45) | 100.31 (96.75, 103.86) | 0.0001 |
| DASH score | |||
| Baseline | 2.895 ± 0.457 | 2.931 ± 0.534 | 0.563 |
| End of trial | 3.837 ± 0.761 | 3.875 ± 0.699 | 0.851 |
| P | < 0.0001 d | < 0.0001 | |
| M1 e | 3.835 (3.616, 4.054) | 3.881 (3.662, 4.100) | 0.771 |
| M 2 | 3.837 (3.626, 4.049) | 3.667 (3.667, 4.090) | 0.787 |
a M1: Model 1 adjusted by age and BMI; M 2: Adjusted by model1 + baseline (dietary intake of energy and macronutrients + fiber + sodium + physical activity). F1: Negative emotions; F2: Availability and physical discomfort; F3: Availability and positive emotions; F4: Social pressure; F5: Disease control and negative emotions. BP systolic: Systolic blood pressure; BP diastolic: Diastolic blood pressure.
b Values are expressed as mean ± SD or mean (95%CI).
c P values denote the significance of between-group differences (P < 0.05, Mann-Whitney U test for non-normal distribution data) and independent t-test (for normal distribution data).
d P values denote the significance of within-group changes (P < 0.05, paired-t test (for normal distribution data) and Wilcoxon test (for non-normal distribution data).
e P value adjusted obtained from ANCOVA.
5. Discussion
The present study revealed that DASH diet education based on the self-efficacy concept for 12 weeks with a smartphone application improved the self-efficacy of subjects and reduced their blood pressure at the end of the intervention compared to the control group.
Improvement of self-efficacy can be useful in the management of chronic diseases (26). People with higher self-efficacy set higher goals, while people with lower self-efficacy have very low expectations for their goals when they try to make changes and tend to surrender when encountering even minute problems (27). All these drawbacks can affect the plans of health staff for improving the health status of patients because they pose a higher risk of failure in the management of chronic diseases (28).
Chronic diseases are complicated and need several positive health behaviors for better control. Previous studies showed that perceived self-efficacy is associated with improvement in different health behaviors in both the short- and long- term which leads to better control of several chronic diseases (28). Higher self-efficacy is also related to more sustainable health behaviors (29). As lifestyle changes and self-efficacy improve, the possibility of management of disease can increase (30, 31).
Different methods have been introduced for better self-efficacy, including the successful performance of assigned tasks, observing others' success with common goals, verbally persuading, and controlling the physiological state of anxiety (28). Different studies evaluated the effect of self-efficacy on changing health behaviors and lifestyles to control chronic diseases. In HTN patients, improvement in self-efficacy showed better management of blood pressure (32).
Dietary approach to stop hypertension diet is one of the most accepted dietary regimens for controlling blood pressure (6). Dietary approach to stop hypertension may act by increasing plasma nitrite, and it can also improve vascular endothelium`s capacity to up-regulate nitric oxide (NO) (33). In fact, due to the high content of nuts, the rich source of L-arginine, it can increase NO as well (34). It can also improve vascular reactivity due to its high antioxidant content. In the present study, we aimed to guide HTN patients in following the DASH diet for three months. All recommendations were delivered through smartphone apps. In recent years, the use of mobile apps for improving health-related problems has grown significantly (35). In essence, using these apps for HTN patients is not an exception. Using smartphone apps showed better drug adherence in a previous trial, which led to controlled blood pressure among those patients (36). Other trials showed similar results, which were improved health status in HTN patients (37). Alessa et al. critically reviewed the possible beneficial effects of using mobile apps in HTN patients. Included studies evaluated the effectiveness of the apps in lowering BP, and more than 70 percent of studies reported that using the apps led to significant decreases in SBP and DBP; authors concluded that these apps may be effective in the self-management of HTN (38). In our study, we showed that using smartphone apps lead to better adherence to the DASH diet, as there was a clinically significant difference between the DASH indexes between the two groups at the end of the study. However, we did not reach statistical significance. In fact, we showed that using this app improved SBP and DBP among HTN patients. In addition, self-efficacy components totally improved at the end of the trial compared with the control group. Indeed, higher self-efficacy leads to better control of BP among patients. Accordingly, in the previous study of elderly subjects in Iran, participants were recruited in motivational classes. At the end of the intervention, the mean of SBP and DBP and also the average scores of social support, self-efficacy, and quality of life improved significantly. However, the study was conducted in a semi-experimental design and without a control group (39). Results were proved in another semi-experimental study on the Korean population. They identified better self-efficacy and self-esteem along with significantly lower SBP and DBP (32). In the present study, a short video of patient experience and speech of an expert dietitian included in the mobile application improved the self-efficacy of patients to better control their systolic and diastolic blood pressure at the end of the study. Similarly, a recent study reported educational video for patients hospitalized with acute ischemic stroke and intracerebral hemorrhage was associated with improved stroke knowledge and self-efficacy in recognizing stroke symptoms (15). A recent study in Iran using mobile application-based- education also showed better adherence to the low-fat and low-salt diet plans among patients with HTN (40).
Another recent study in Iran concluded that designing and implementing educational programs that address self-efficacy and perceived susceptibility and severity of hypertension may lead to improvements in self-care behaviors (41).
Other cross-sectional studies also evaluated the possible association between self-efficacy and self-care management of HTN. In this regard, a previous study showed that better self-efficacy was not only associated with increased adherence to medications but also led to the consumption of a low-salt diet and practicing weight management skills among African-American patients with HTN (42).
5.1. Strengths and Limitations
The present study has some limitations that need to be acknowledged. In this study, people of different ethnicities in Khuzestan province were included. It was not possible to increase the sample size, make it more generalizable, and consider the differences between ethnicities to educate the DASH regime and evaluate self-efficacy. The other limitation was our failure to measure the metabolic profile. However, we assigned the sample selection among the general population referring to public health care centers in Ahvaz city.
In addition, the application was designed only for Android devices as Android was more affordable, and having access to an Android device would also be possible for a person with an iPhone.
Furthermore, this is the first study to provide an offline application based on self-efficacy with a short video of the patient's satisfactory experiences following the DASH diet. She was then preparing a short video of a dietetics specialist in the form of a keynote lecture on the importance of the DASH diet and a healthy heart. According to research evidence, educational videos can be more effective than written material at increasing knowledge and modifying health behaviors (15).
In addition, they provide information in a divided section on the features of this diet in relation to weight control, blood pressure, blood lipid, and sugar. Therefore, future long-term studies with large-scale designs are needed before a confirmed conclusion can be drawn.
5.2. Conclusions
We observed that using a mobile app for adhering to the DASH diet and improving self-efficacy led to better control of BP among patients while their self-efficacy improved simultaneously. However, future studies are required to confirm the results of the present study.