1. Background
Chronic kidney disease (CKD) includes a group of heterogeneous disorders that affect the structure and function of the kidney (1). The prevalence of this disease is estimated to be 10 - 13% worldwide and 15% in Iran (2).
Chronic kidney disease can negatively affect the patients' social, financial, and psychological well-being (3). Patients with CKD are prone to emotional problems for various reasons, including chronic stress related to the burden of the disease, dietary restrictions, functional limitations, related chronic diseases, and adverse effects of medications. The emotional problems of these patients include depression, stress, and anxiety. The diagnosis and treatment of these disorders can improve their quality of life and reduce their mortality risk (4). According to meta-analyses, the prevalence of depression in patients with CKD is about 21 - 27%, and the prevalence of anxiety in them is 19 - 43% (5, 6). Depression in patients with CKD is associated with poorer health status, decreased quality of life, and increased healthcare utilization (7). Various factors can play a role in causing anxiety in patients with CKD, including inflammation, oxidative stress, and renin-angiotensin system involvement (8).
Evidence shows that a high-quality diet and its components, as well as nutritional supplements, can help prevent or treat stress, anxiety, and depression (9). To the best of our knowledge, very few studies have investigated the relationship between dietary intake, stress, anxiety, and depression in patients with CKD.
2. Objectives
Since the prevalence of stress, anxiety, and depression in these patients seems to be high and diet plays an important role in the prevention or treatment of these disorders, the present study aimed to evaluate the correlation of dietary intake with stress, anxiety, and depression in patients with CKD.
3. Methods
3.1. Study Population
This was a cross-sectional study, and the participants were patients with CKD visiting the Kidney Diseases Clinic affiliated with Kashan University of Medical Sciences (Iran) from 2021 to 2022. The simple random sampling method was used. Those who were included in the study were all over 18 years old; according to the nephrologist's diagnosis, they were in stages 2 to 5 of CKD and had an estimated glomerular filtration rate (eGFR) of less than 90 mL/min/1.73 m2. The other criteria for inclusion were: (1) Not suffering from cancer, lung diseases, mental retardation, or dementia; (2) absence of pregnancy and breastfeeding; (3) not receiving immunosuppressive medications; (4) absence of hemodialysis, peritoneal dialysis, and kidney transplantation. The exclusion criteria were: (1) عnwillingness to participate in the study; and (2) incomplete filling of the questionnaires.
At the beginning of the study, the objectives and method of the study were explained to the patients, and then they all signed the written informed consent form. All the stages of the research were approved by the Ethics Committee of Kashan University of Medical Sciences (code of ethics: IR.KAUMS.MEDNT.REC.1400.065). The present study is a secondary analysis of the previous research. The details of the study and the method of sample size calculation were explained elsewhere (10). Briefly, a minimum of 80 subjects was needed to detect a significant difference in correlation coefficients between serum zinc and diastolic blood pressure (DBP), where the alpha parameter is 1% and the beta parameter is 2%. After obtaining written informed consent from the patients, the researchers filled out a general questionnaire to collect the patients' demographic data (age, sex, education, marital status, employment), history of diseases, and the use of medications and nutritional supplements. Height, weight, and body mass index (BMI) were the other variables evaluated in the present study. Estimated glomerular filtration rate values in the present study were calculated using the CKD-EPI (chronic kidney disease-epidemiology collaborative group) formula (11).
3.2. Nutritional Assessments
The researchers completed 3 days of 24-hour food recall (1 holiday and 2 work days) through face-to-face and telephone interviews to assess the patients' diet. The dietary data analysis of the 24-hour food recall questionnaires was performed using the Nutritionist IV (N4) software.
3.3. Assessment of Stress, Anxiety, and Depression
The short self-report validated Iranian version of the Depression, Anxiety, and Stress Scale 21 (DASS-21) was used to investigate psychological problems, including depression, anxiety, and stress (12). After reading the statements in the questionnaire, the participants gave their immediate answers based on a Likert scale ranging from 0 (does not apply to me at all) to 3 (applies to me a lot or most of the time). Since DASS 21 is a short version of DASS-42 (a 42-question self-report questionnaire), the summed scores in each subscale should be multiplied by 2. Therefore, in the present study, depression, anxiety, and stress scores were expressed as ≥ 10, ≥ 8, and ≥ 15, respectively.
3.4. Statistical Analysis
Frequency distribution tables and graphs were used to present qualitative variables, and central and dispersion indices were used to present quantitative variables. Pearson and Spearman correlation coefficients were utilized to check the correlation between food intake and stress, anxiety, and depression scores. Multivariate linear regression was used to assess the corresponding associations while controlling for confounding variables (age, sex, energy, BMI, diabetes). The data were analyzed using SPSS v. 16 software (IBM Corp., Armonk, NY, USA), and the significance level was considered P < 0.05.
4. Results
The researchers evaluated 2,500 files of CKD patients according to the inclusion and exclusion criteria at the beginning of the study to collect the desired samples. After checking the files and calling the target patients, 2 410 were not included since they met the exclusion criteria or were unwilling to participate. Finally, 90 patients with CKD were included.
Demographic and other characteristics of the patients with CKD are presented in Table 1. Out of 90 patients studied, 64 were men, and 26 were women; the age range of the participants was 34 to 76 years, and their average age was 60.68 years. The prevalence of stress, anxiety, and depression was estimated to be 60.7%, 54.1%, and 54%, respectively. The distribution of the participants regarding the severity of depression, anxiety, and stress is shown in Table 2.
| Characteristic | Values (n = 90) |
|---|---|
| Age (y) | 60.68 ± 8.81 |
| Male (%) | 71.1 |
| BMI, kg/m2 | 30.47 ± 5.65 |
| Job history (%) | |
| Homemaker | 27.8 |
| Employed | 28.9 |
| Retired | 42 |
| Unemployed | 1.1 |
| Smoking (%) | 16.7 |
| Education history (%) | |
| Illiterate | 12.2 |
| Elementary | 46.7 |
| Junior high school | 10 |
| High school diploma | 24.4 |
| Associate degree | 2.2 |
| Bachelor's degree and above | 4.4 |
| History of diseases (%) | |
| Cardiovascular diseases | 35.6 |
| Hyperlipidemia | 75.6 |
| Diabetes | 61.1 |
| Kidney stones | 24.4 |
| eGFR (mL/min/1.73 m2) | 43.23 ± 17.19 |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate.
a Data are expressed as the mean ± standard deviation or percentage.
| No. (%) | |
|---|---|
| Depression | |
| Normal | 40 (46) |
| Mild | 9 (10.3) |
| Moderate | 16 (18.4) |
| Severe | 12 (13.8) |
| Very severe | 10 (11.5) |
| Anxiety | |
| Normal | 39 (45.9) |
| Mild | 10 (11.8) |
| Moderate | 14 (16.5) |
| Severe | 4 (4.7) |
| Very severe | 18 (21.2) |
| Stress | |
| Normal | 33 (39.3) |
| Mild | 15 (17.9) |
| Moderate | 12 (14.3) |
| Severe | 17 (20.2) |
| Very severe | 7 (8.3) |
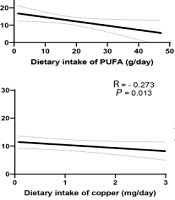
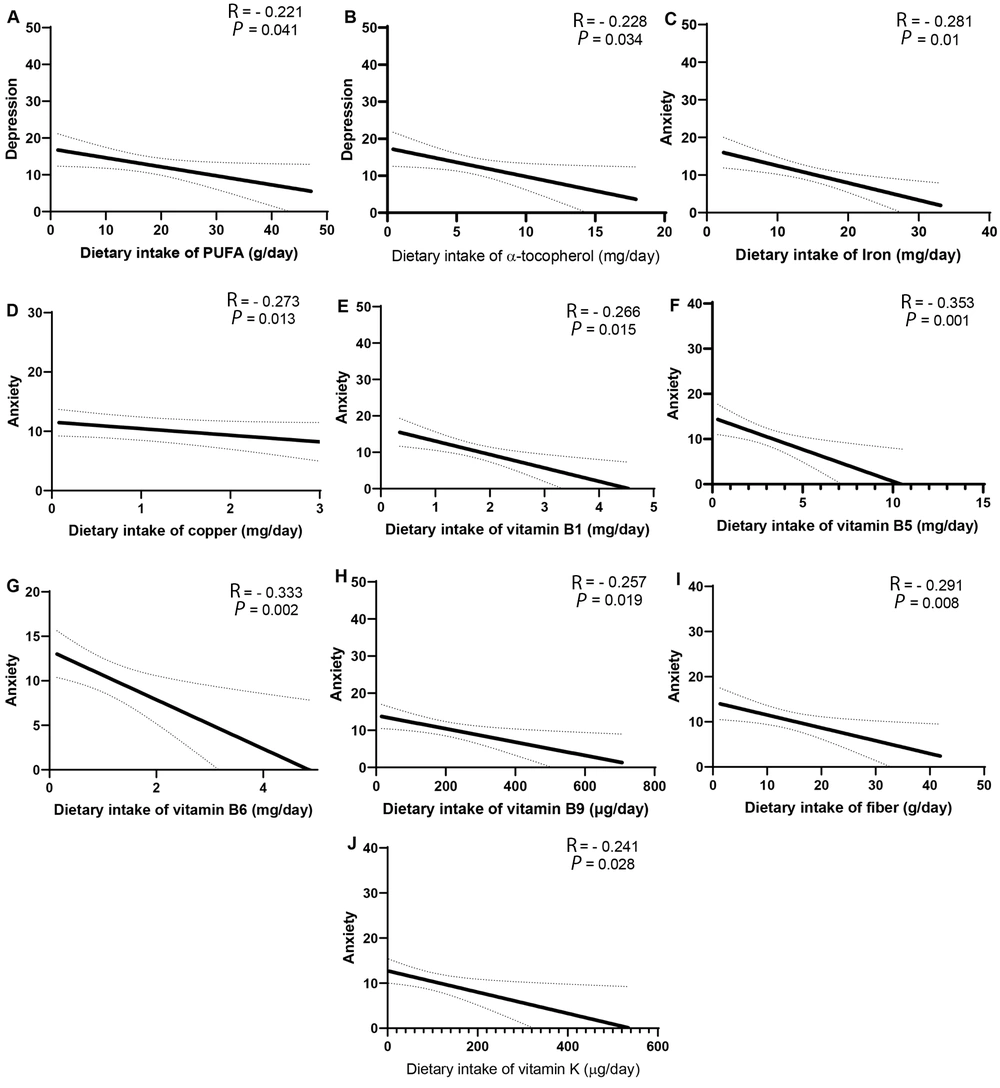
Based on Figure 1, there was a significant inverse correlation between depression and the dietary intake of polyunsaturated fatty acids (PUFAs) and α-tocopherol. In addition, a significant inverse correlation was found between anxiety and the dietary intake of iron, copper, B vitamins (B1, B5, B6, B9), fiber, and vitamin K. After adjusting for confounding variables such as age, sex, energy, BMI, and diabetes mellitus, the association between depression and the dietary intake of PUFAs (β = - 0.214, P = 0.039) and α-tocopherol (β = - 0.225, P = 0.025), as well as the association between anxiety and the dietary intake of iron (β = - 0.319, P = 0.003), copper (β = - 0.25, P = 0.031), vitamin B1 (β = - 0.314, P = 0.004), vitamin B5 (β = - 0.262, P = 0.016), vitamin B6 (β = - 0.292, P = 0.007), vitamin B9 (β = - 0.241, P = 0.026), vitamin K (β = - 0.26, P = 0.015), and fiber (β = - 0.224, P = 0.04) remained significant.
Association between depression and dietary intake of polyunsaturated fatty acids (A); association between depression and dietary intake of α-tocopherol (B); association between anxiety and dietary intake of iron (C); association between anxiety and dietary intake of copper (D); association between anxiety and dietary intake of vitamin B1 (E); association between anxiety and dietary intake of vitamin B5 (F); association between anxiety and dietary intake of vitamin B6 (G); association between anxiety and dietary intake of vitamin B9 (H); association between anxiety and dietary intake of fiber (I); association between anxiety and dietary intake of vitamin K (J).
5. Discussion
A significant inverse correlation was obtained between depression and the dietary intake of PUFAs and α-tocopherol. Moreover, a significant inverse correlation was found between anxiety and the dietary intake of iron, copper, vitamin B1, vitamin B5, vitamin B6, vitamin B9, vitamin K, and fiber.
The prevalence of stress, anxiety, and depression was estimated to be 60.7%, 54.1%, and 54%, respectively. Aggarwal et al. aimed to determine the prevalence of anxiety, depression, and insomnia in patients with CKD in India (n = 200); they estimated the prevalence of anxiety and depression to be 71% and 69%, respectively. The rate of mood disorders obtained in their studies was close to the values found in the present research (4).
One of the results of the present study was the presence of a significant inverse correlation between dietary intake of PUFAs and depression in patients with CKD. Similar to our result, in the study of Panagiotakos et al. on the relationship between fatty acid intake and depression symptoms in Greece, an inverse and nearly significant relationship was observed between PUFA intake and depression symptoms (P = 0.06) (13). The mechanism through which PUFAs can affect depression might be through their effect on inflammatory processes related to depression (14). For example, evidence from human studies revealed that supplementation with eicosapentaenoic acid and docosahexaenoic acid could reduce depression symptoms by suppressing the production of interleukin-1 (IL-1), interleukin-2 (IL-2), interleukin-6 (IL-6), and tumor necrosis factor α (TNF-α) (15).
Another result of the present study was the presence of a significant inverse correlation between the dietary intake of α-tocopherol and depression in patients with CKD. In line with our result, in the study of Nguyen et al., a significant negative relationship was observed between the intake of α-tocopherol from the diet and depressive symptoms in Japanese older adults (16). In a meta-analysis study by Lee et al., a significant lowering effect of α-tocopherol supplementation on depression was found (17). Based on the previous evidence, it has been suggested that vitamin E can play a role in reducing the symptoms of depression through its antioxidant and anti-inflammatory properties (17). Vitamin E can play a role in reducing depression by decreasing lipid peroxidation and superoxide production, disrupting the formation of foam cells, and reducing the secretion of pro-inflammatory cytokines such as interleukin-8 (18).
The current study observed a significant negative association between dietary iron intake and anxiety in patients with CKD. Similarly, in the study of Chen et al., a significant direct relationship was obtained between iron deficiency anemia and anxiety in children and adolescents (19). Based on the results of previous animal studies, iron deficiency anemia may affect diseases such as anxiety through changes in serotonin, norepinephrine, and gamma amino butyric acid (GABA) neurotransmission (20). In Beard et al.'s study, weaned mice that were fed an iron-deficient diet for 6 weeks showed an increase in anxiety-like behaviors (21).
In addition, we found a significant inverse relationship between dietary copper intake and anxiety in patients with CKD. Mieko Nakamura et al. also observed a significant negative correlation between the dietary intake of copper and symptoms of anxiety and depression in Japan (22). Copper might decrease anxiety through various mechanisms, including its beneficial roles in modulating the glutamatergic, monoaminergic, and GABAergic systems and via reducing the oxidative stress due to its role in the activity of superoxide dismutase (23).
B vitamins are among the other nutrients that have shown evidence of effects on mental and mood states. In the present study, we observed a significant negative association between the dietary intake of vitamins B1, B5, B6, and B9 and the anxiety score in patients with CKD. Similar to our results, Mahdavifar et al. found an inverse relationship between the intakes of vitamin B1 and B5 and anxiety symptoms (24). B vitamins act as co-enzymes in the body and, by regulating the proper functioning of the methylation cycle, might have beneficial effects on producing the neurotransmitters. In addition, B vitamins can protect against hyperhomocysteinemia, which has deleterious effects on neural and mental health (25).
The present study results showed that a lower intake of vitamin K was associated with a higher anxiety score. So far, human studies have not investigated the relationship between vitamin K and mood states. However, in an animal study by Gancheva and Zhelyazkova-Savova the intake of vitamin K2 by rats with metabolic syndrome reduced anxiety. They proposed that the beneficial effect of vitamin K on anxiety might be due to its ability to lower blood glucose levels, which is related to anxiety disorders (26).
Finally, we found that increased dietary fiber was significantly correlated with a lower level of anxiety. Similarly, in a cross-sectional study by Liu et al. conducted on 459 subjects with hypertension, a higher dietary fiber was associated with lower anxiety (27). Fibers can reduce anxiety symptoms in humans through various mechanisms, including regulating inflammatory factors and neurotransmitters (28). For example, it has been stated that galacto-oligosaccharides, as dietary fibers, can reduce the expression of inflammatory factors such as interleukin-1β (IL-1β), IL-6, and TNFα through the production of acetic acid. In addition, dietary fibers may increase neurotransmitters such as glutamate and gamma-aminobutyric acid levels in the hypothalamus and ultimately alleviate anxiety symptoms in humans (28).
The present study had some limitations that need to be addressed. It was cross-sectional, which made it impossible to determine whether there were any cause-effect relationships. There was also a limitation in the dietary intake assessment methodology since it relied on the subjects' ability to recall the food they ate within the last 24 hours, which limits the accuracy of the data that could be obtained about dietary intake. Despite this, we decided to conduct 3-day food recalls (2 weekdays and 1 weekend) to improve the accuracy of estimating the daily intake of food. Most of the study subjects were men, which can affect the generalizability of the results.
Overall, the lack of B vitamins, vitamin K, iron, copper, and dietary fiber might be related to the increase in depression and anxiety in patients with CKD. It should be noted that these patients are highly exposed to the lack of mentioned nutrients due to the restrictions they apply in their diet to control the progress of the disease (29). Examples of dietary restrictions in these patients include reducing the intake of protein food sources (such as meats and eggs), potassium (such as vegetables and fruits), and phosphorus (such as cereal bran, legumes, and nuts), which provide B vitamins, vitamin K, iron, chromium, and dietary fiber (30, 31). Therefore, it seems that food enrichment or supplementation in these patients might help improve depression and anxiety and increase the quality of life. However, the findings of longitudinal studies and clinical trials will reveal more evidence in this regard.
5.1. Conclusions
Based on the current study results, the dietary intake of nutrients such as PUFAs, α-tocopherol, B vitamins, vitamin K, iron, copper, and fiber might be associated with depression and anxiety. However, further studies with longitudinal designs are needed to reveal the cause-effect relationship in this regard.