1. Background
Depression is a common comorbidity in adult patients with type 2 diabetes mellitus (DM) (1-3). In the United States, 28% of women and 18% of men with DM suffer from depressive symptoms (4). In Iran, one-third of patients with DM have been identified to have depression (5). Compared with the general population worldwide, the incidence of depression is two times higher in patients with DM (4, 6-8). A bidirectional relationship has been found between depression and DM (9, 10). Poor mental health and disease-related complications are more prevalent in patients with diabetes and comorbid depression. Patients with diabetes and depression might experience more difficult glycemic control, poorer quality of life, and low adherence to self-care behaviors. In addition, depression may predict a higher incidence of vascular complications in DM patients and can increase the morbidity and mortality rates in these patients (11, 12).
Different pharmacological, psychological, or combined therapeutic approaches have been introduced for diabetic patients with depression (4, 13). Selective serotonin reuptake inhibitors (SSRIs) are among antidepressants that have been reported to inhibit insulin secretion by affecting pancreatic cells. Accordingly, there are disagreements over the treatment of depression in DM patients using these drugs, with some papers reporting an increase in plasma glucose in patients treated with SSRIs, an observation that has been debated by some other studies (14-16).
Despite growing evidence on the prevalence and importance of depression in patients with DM, there is little research comparing the effectiveness, tolerability, side effects, and cross-reactions of different medications used to manage depressive disorders in patients with DM (17, 18). SSRIs have been introduced as the gold-standard treatment for depression in patients with diabetes. These medications have good tolerability; however, limited evidence is available on the superiority of any specific type of SSRIs (14, 19).
2. Objectives
Considering the controversies regarding the effectiveness of different antidepressants in adult patients with comorbid diabetes and depression, this research aimed to compare the effectiveness and safety of two SSRIs (sertraline and fluoxetine) in these patients.
3. Methods
3.1. Trial Design and Participants
This parallel-designed randomized controlled trial was conducted on adult patients (aged 18 years and over) with type 2 diabetes referring to the endocrine clinic affiliated with the Babol University of Medical Sciences, Iran, whose DM diagnosis was confirmed by an endocrinologist. Consequently, a structured psychiatric clinical interview was used to affirm the presence of a depressive disorder according to DSM-IV criteria.
Inclusion criteria were an age of 18 years and over, the confirmation of DM diagnosis by an endocrinologist, and the presence of depressive symptoms affirmed in a structured psychiatric clinical interview.
Pregnancy and breastfeeding, immigration during the study, the presence of uncontrolled cardiovascular disease, ophthalmic disorders, and a history of mood disorders have been considered as exclusion criteria.
3.2. Interventions
The participants were distributed in two groups via the simple random allocation method. The first group received 50 - 200 mg/day of sertraline, and the second group received 20 - 60 mg/day of fluoxetine. Both of these drugs were manufactured by Abidi´s pharmaceutical company, Iran.
In the sertraline group, the intervention was initiated with 50 mg/day, and the dose increased up to 200 mg/day depending on the patient’s response and decrease in depressive symptoms during monthly visits. In the fluoxetine group, the medication was started at the dose of 20 mg/day and scaled up to 60 mg/day depending on the patient’s response and improvement in depressive symptoms.
The patients in the two groups received the medications for 12 weeks. Demographic characteristics, including age, gender, occupation, marital status, education level, the condition of comorbid illnesses, smoking, the time spent for physical activity in the previous week, and currently in-used medications were recorded. In addition, a physical examination was conducted to determine the patient’s height, weight, body mass index (BMI), and blood pressure. Fasting blood sugar (FBS), blood glucose 2 hours after a meal, HbA1c level, and serum lipid profile (triglycerides, cholesterol, HDL, and LDL) were measured at the baseline and 12 weeks after the intervention. The participants of both groups were informed about their access to a consultant psychiatrist during the intervention in order to eliminate the effects of depression on blood glucose and other research outcomes.
The Beck Depression Inventory-II (BDI-II) was used to examine the severity of depressive symptoms in patients at the baseline and 12 weeks after the intervention. This patient-rated questionnaire contains 21 items assessing symptoms and attitudes, including the cognitive, affective, somatic, and vegetative symptoms of depression. The items of BDI-II are rated on a 4-point scale ranging from 0 to 3 based on the severity of symptoms, with a maximum total score of 63. The score ranges of 0 - 13, 14 - 19, 20 - 28, and 29 - 63 indicate minimal, mild, moderate, and severe depression, respectively (20).
3.3. Outcomes
Primary outcomes included the BDI-II score and glucose profile (FBS, 2-hour post-prandial blood glucose, and HbA1c), and secondary outcomes included BMI, serum lipid profile, and drug side effects.
3.4. Sample Size
The sample size in each group was calculated as n = 16 (based on a 95% confidence interval, 80% study power, and the assumption that σ1 = σ2 = 1 for finding 1 unit of difference in HbA1c between the two groups after the intervention). Regarding the probable 20% loss to follow-up, the sample size was calculated as n = 20 in each group.
3.5. Randomization
Eligible people were allocated to one of the two intervention groups of A (n = 20) and B (n = 20) using the permuted block randomization method with 10 blocks. The size of the blocks was four (AABB) to generate possible sequences.
3.6. Statistical Methods
The data were analyzed using SPSS version 22 software. The chi-square test was used to compare qualitative variables between the study groups, and covariance analysis (ANCOVA) was used to compare the impact of the interventions in the two groups. A P-value less than 0.05 was designated as the statistical significance level.
3.7. Ethical Consideration
The patients were assured that their information would remain confidential; they received free-of-charge visits and could leave the study whenever they wanted, while this decision was not a barrier to receiving other health services. This study was approved by the Ethics Committee of Babol University of Medical Sciences, Iran, under the reference number of MUBABOL.REC.1396.16. The study protocol was also registered on the website of the Iranian Registry of Clinical Trials (www.irct.ir) with the ID IRCT20150630022991N9.
4. Results
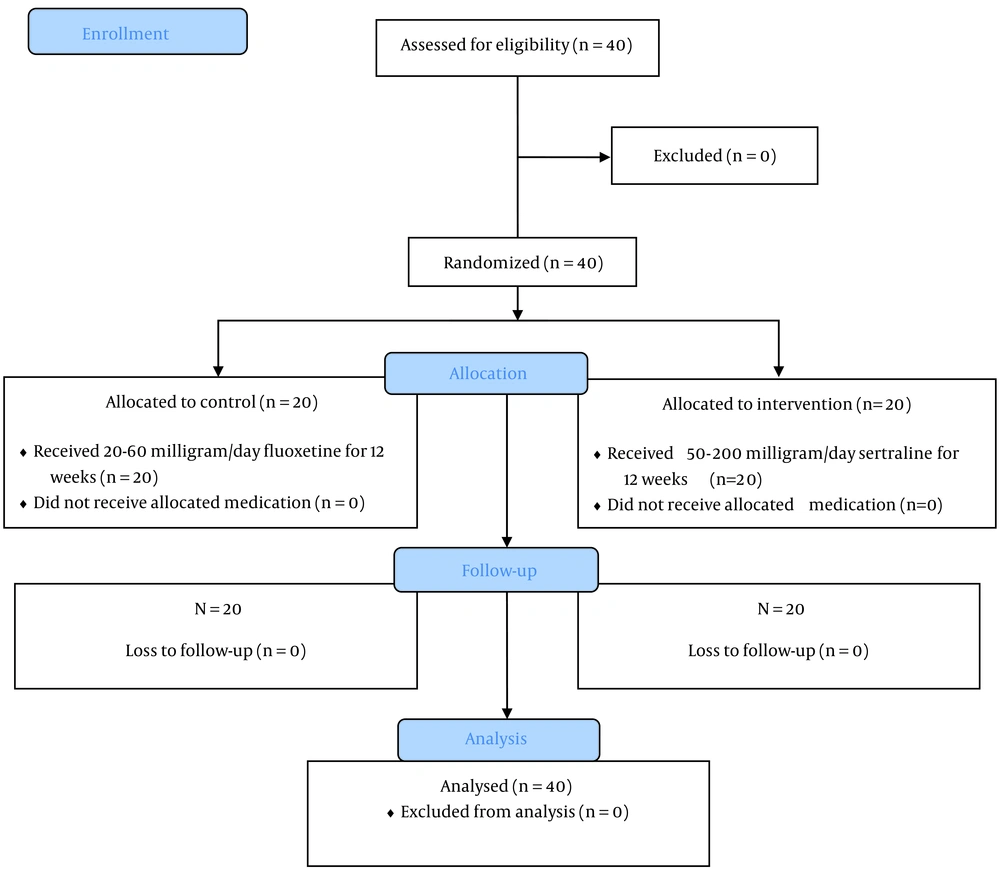
In total, 40 patients with DM and depressive symptoms were enrolled in this study: 20 individuals in the intervention group (sertraline) and 20 in the control group (fluoxetine). The flow diagram of the participant enrollment process has been presented in Figure 1. The baseline characteristics of the patients in the intervention and control groups have been presented in Table 1. None of the examined demographic characteristics, lifestyle behaviors (smoking and physical activity), and medical conditions (type of diabetes treatment, duration of DM, diabetes-related complications, and history of hospitalization) were significantly different between the two groups at the baseline (P > 0.05).
| Characteristics | Sertraline Group (N = 20) | Fluoxetine Group (N = 20) | P-Value |
|---|---|---|---|
| Age group, y | 0.67 | ||
| 30 - 45 | 4 (20) | 6 (30) | |
| 45 - 60 | 10 (50) | 10 (50) | |
| >60 | 6 (30) | 4 (20) | |
| Age, mean ± SD | 55.0 ± 10.7 | 52.2 ± 12.4 | 0.44 |
| Gender | 0.29 | ||
| Female | 19 (95) | 17 (85) | |
| Male | 1 (5) | 3 (15) | |
| Living region | 0.73 | ||
| Urban | 6 (30) | 7 (35) | |
| Rural | 14 (70) | 13 (65) | |
| Education level | 0.99 | ||
| Lower than diploma | 18 (90) | 18 (90) | |
| Diploma and higher education | 2 (10) | 2 (10) | |
| Occupation | 0.27 | ||
| Housekeeper | 18 (90) | 16 (80) | |
| Others | 2 (10) | 4 (20) | |
| Marital status | 0.89 | ||
| Married | 16 (80) | 17 (85) | |
| Divorced | 1 (5) | 1 (5) | |
| Widow | 3 (15) | 2 (10) | |
| Smoking | 0.07 | ||
| Yes | 0 (0) | 3 (15) | |
| No | 20 (100) | 17 (85) | |
| Physical activity (min/w) | 0.31 | ||
| Less than 600 | 8 (40) | 5 (25) | |
| ≥600 | 12 (60) | 15 (75) | |
| Duration of diabetes diagnosis, y | 0.74 | ||
| 1 - 10 | 12 (60) | 13 (65) | |
| >10 | 8 (40) | 7 (35) | |
| Mean ± SD | 9.5 ± 8.1 | 10.2 ± 9.5 | 0.80 |
| Type of diabetes treatment | 0.90 | ||
| Oral medication | 10 (50) | 9 (45) | |
| Insulin therapy | 3 (15) | 4 (20) | |
| Oral + insulin therapy | 7 (35) | 7 (35) | |
| Previous history of hospitalization | 0.75 | ||
| Yes | 10 (50) | 11 (55) | |
| No | 10 (50) | 9 (45) | |
| Diabetes-related complications | 0.70 | ||
| Yes | 16 (80) | 15 (75) |
Baseline Demographic Features, Lifestyle Behaviors, and Medical Profile of the Study Participants
Table 2 summarizes physical symptoms, laboratory parameters, and depression severity in the two groups before and after the intervention. Body mass index, systolic blood pressure, FBS, 2-hour post-prandial blood glucose, HbA1c, serum levels of total cholesterol, triglyceride, LDL cholesterol, and depression severity score showed reductions to lower values after the intervention compared to the baseline in both intervention and control groups; however, no statistically significant difference was observed between the two groups (P > 0.05).
| Variables | Before Intervention, Mean ± SD | After Intervention, Mean ± SD | P-Value | ||
|---|---|---|---|---|---|
| Sertraline Group | Fluoxetine Group | Sertraline Group | Fluoxetine Group | ||
| Body mass index, kg/m2 | 28.9 ± 5.0 | 27.7 ± 4.2 | 28.1 ± 5.0 | 26.1 ± 3.9 | 0.40 |
| Systolic blood pressure, mmHg | 115.8 ± 19.6 | 122.3 ± 19.9 | 114.5 ± 17.6 | 121.3 ± 17.3 | 0.41 |
| Diastolic blood pressure, mmHg | 65.0 ± 8.7 | 70.3 ± 10.7 | 65.3 ± 8.3 | 68.3 ± 12.3 | 0.15 |
| Fasting blood glucose | 164.6 ± 64.5 | 172.1 ± 64.8 | 138.2 ± 32.5 | 148.7 ± 49.9 | 0.46 |
| 2 hour post-prandial blood glucose | 217.7 ± 73.0 | 232.8 ± 72.3 | 191.8 ± 52.5 | 194.6 ± 62.1 | 0.53 |
| HbA1c | 8.1 ± 1.4 | 8.5 ± 1.7 | 7.4 ± 0.9 | 7.7 ± 1.2 | 0.59 |
| Serum total cholesterol, mg/dL | 175.9 ± 45.2 | 188.1 ± 70.3 | 169.9 ± 44.2 | 178.7 ± 64.0 | 0.78 |
| Serum triglyceride, mg/dL | 203.2 ± 89.9 | 197.2 ± 85.4 | 176.1 ± 57.3 | 192.5 ± 82.0 | 0.04 |
| Serum LDL cholesterol, mg/dL | 89.6 ± 23.1 | 99.4 ± 30.9 | 87.6 ± 23.4 | 97.3 ± 26.2 | 0.62 |
| Depression severity score, based on BDI-II | 32.4 ± 7.3 | 29.6 ± 4.8 | 16.8 ± 9.1 | 18.0 ± 11.6 | 0.27 |
Patients’ Physical Examination Findings, Laboratory Parameters, and Depression Severity Score Before and After the Interventions
Covariance analysis for systolic and diastolic blood pressure, FBS, 2-hour post-prandial blood glucose, HbA1c, serum levels of total cholesterol, triglyceride, and LDL cholesterol, and the depression severity score revealed that sertraline could significantly reduce serum triglyceride compared to fluoxetine (P = 0.04). Regarding other variables, this difference was not statistically significant (P > 0.05). In addition, covariance analysis for age (P = 0.45), gender (P = 0.54), and duration of diabetes (P = 0.36) revealed no significant difference between the sertraline and fluoxetine groups (P > 0.05).
The most common drug side effects were reported in weeks 4th and 8th after treatment and included decreased appetite, nausea, and loss of libido.
5. Discussion
Our results showed that both sertraline and fluoxetine had a positive impact on depression symptoms in DM patients, evidenced by a reduction in the depression severity score. This observation indicated that these two SSRIs were effective in the control of blood glucose in our patients, confirmed by a decrease in HbA1c, FBS, and 2-hour post-prandial blood glucose. Roopan and Larsen studied the effects of antidepressants on patients with depression and comorbid DM in a systematic review of randomized controlled double-blinded trials investigating the therapeutic effects of antidepressants. In the recent study, all of the reviewed trials reported a reduction in depressive symptoms after treatment with an antidepressant drug in both the acute and maintenance phases. This study showed that an improvement in depression symptoms could have a positive effect on glycemic control independently of the patient's weight, suggesting the beneficial effects of SSRIs in treating depressive symptoms in patients with DM (21).
Komorousova et al. emphasized that the treatment of depressive disorders in DM patients using antidepressants could have a favorable effect not only on patients’ mental status and treatment compliance but also on their glycemic control (22). Radojkovic recruited 58 patients with poorly controlled DM whose BDI-II score was ≥14 with a confirmed diagnosis of depression and reported that 6-month treatment with SSRI antidepressants led to a positive linear correlation between depression improvement and glycemic control (23). Gehlawat et al. studied the effects of Escitalopram (an SSRI) on 40 depressed patients with DM and reported that depressive symptoms subsided significantly after three weeks of treatment, accompanied by a decline in fasting and post-prandial blood glucose levels in weeks 6th and 12th of treatment, respectively, as well as a reduction in HbA1c level in week 12th after the intervention (24). Petrak et al. compared the efficacy of cognitive behavioral group therapy and sertraline in patients with DM and depression and found that 45.8% of the patients positively responded to antidepressant treatment. The comparison of HbA1c levels revealed no significant difference between these two groups, and depression symptoms improved in both groups with a significant advantage for sertraline (25). The co-occurrence of DM and depression significantly worsens the prognosis of both diseases and increases patients’ morbidity and mortality. Furthermore, up to 25% of DM patients may experience depression symptoms; however, the severity of these symptoms does not reach the threshold for the diagnosis of the disorder. This can delay the timely diagnosis of depression in DM patients, and delayed treatment can cause unwanted side effects (26, 27).
Salvi et al. evaluated the risk of new-onset diabetes in antidepressant users in a systematic review and meta-analysis and debated whether a single antidepressant could have an effect on the risk of diabetes, suggesting that the classification of antidepressants according to their pharmacological profiles could be useful in better understanding of the nature of this association (28).
Barnard et al. reviewed three systemic reviews and 22 studies to divulge the association of type 2 DM with the use of antidepressants. They declared that the use of antidepressants could be associated with type 2 diabetes; however, a causal link was not established; rather, a complex association was suggested between consuming antidepressants and DM development. Some antidepressants have been linked to worsening glycemic control, particularly at higher doses and in longer durations. On the other hand, some other studies have reported improved glycemic control in diabetic consumers of antidepressants. More recent larger studies have suggested a modest effect, arguing that despite evidence suggesting antidepressant use as an independent risk factor for DM, long-term longitudinal studies are required to scrutinize the effects of individual antidepressants, rather than drug classes, on the risk of DM (29).
In the current study, post-prandial blood glucose level, mean body weight, and BMI reduced to a larger extent in patients who received fluoxetine than in those treated with sertraline 12 weeks after treatment; however, the differences were not statistically significant. Another important finding in this study was a significant reduction in serum triglyceride levels in the sertraline group. In contrast to our finding, in Radojkovic et al.’s study, 6-month treatment with SSRIs was found to have positive effects on glycemic control but not on lipid profile in DM patients with comorbid depression (23). Antidepressants can induce weight gain. Kivimaki et al. reported that SSRI use, despite being related to weight maintenance or even weight loss in the short term, might be associated with an increased risk of weight gain in the long term. It is notable that overweight and abdominal fat mass are determinants of insulin resistance, so antidepressants that induce weight gain should be prescribed with caution in patients at risk of diabetes (30). In a placebo-controlled pre-clinical trial, Silverstein-Metzler studied the effects of the long-term use (18 months) of SSRIs on body composition and carbohydrate metabolism and concluded that sertraline might have beneficial effects on body composition and carbohydrate metabolism but deleterious effects on adiponectin, a cardiovascular risk factor (31).
Recent studies have recommended combining patient education with either psychotherapy or pharmacotherapy in patients with comorbid DM and depression to achieve better self-care and glucose control outcomes, as well as to implement a national or regional integrated health system to follow up diabetic patients and monitor their adherence to therapeutic regimens (26).
Considering that short-term treatment with fluoxetine and sertraline had a positive impact on depression symptoms and glycemic control in our patients, we suggest that these drugs can be used in adult patients with comorbid depression and type II diabetes mellitus. However, in the case of long-term treatment with higher daily doses of these drugs, regular and meticulous monitoring of therapeutic outcomes, especially anthropometric measures and cardiovascular risk factors, is recommended.
The most important limitation of this study was the lack of adjustment for diabetes medications or possible nutritional supplements received by patients in the two groups. Also, we did not consider the family history of diabetes and depression in this research. For future studies, anti-diabetes drugs and daily food intake, as well as the family history of DM and depression, are recommended to be taken into account.
5.1. Conclusions
Three months of treatment with either sertraline or fluoxetine had a positive impact on depression symptoms and decreased HbA1c, FBS, and 2-hour post-prandial blood glucose in adult patients diagnosed with comorbid depression and type II diabetes mellitus. Health service providers are recommended to regularly monitor glucose profile and cardiovascular risk factors in patients with diabetes and depression receiving long-term treatment with SSRIs.