1. Background
As an increasing global health burden, cancer is the leading cause of death in the developed world and the second-leading cause in the developing world (1). A review of the global prevalence of oral cancer reveals a wide variation in distribution among countries. Still, two-thirds of the estimated incidence of oral cancer occurred in developing countries (2). 2018, the International Agency for Research on Cancer (IARC) reported approximately 18.1 million new cancer cases and 9.5 million related deaths worldwide. During the same year, 354,864 new cases of oral and oropharyngeal cancers were reported, representing 2% of all cancer cases (2.9% of all cancer cases among men and 1.0% among women globally) (3). One of the most important types of cancers is oral and oropharyngeal cancer, which ranks as the sixth most prevalent cancer and is known as one of the few lethal oral diseases with a great burden associated with a high cost of treatment and rehabilitation, life-long impediments, and nearly 50% mortality rate (4, 5). Therefore, it should be considered an increasing and serious health and social problem in the world, considering the increase in new cases by nearly 42% from 2020 to 2040, according to the Global Cancer Observatory (GCO) projections. As reported in estimates, the incidence rate of oral and oropharyngeal cancer is also increasing in Asia by 45.7%. Iran, as one of the Asian nations with a high human development index (HDI), tends to have higher rates of incidence due to associated risk factors. Although the GLOBCAN 2018 data shows lower ASR in comparison with most of the Asian nations (3), it should not be overlooked that the estimates of GCO for oral and oropharyngeal cancer show a 100% increase in cases by 2040 in Iran.
Oral and oropharyngeal cancers include cancer of the tongue, lip, the floor of the mouth, gingiva, palate and buccal mucosa, alveolar mucosa, and oropharynx, as well as the pharyngeal tonsils and salivary glands [ ICD-10: C00-14] (3, 4). Ninety percent of oral cavity cancers are squamous cell carcinomas (SCC), and other types of oral cavity cancers such as minor salivary gland malignancies, sarcomas, malignant odontogenic tumors, melanoma, and lymphoma comprise less than 10% of oral cavity cancers (6). Due to the rare nature of these malignancies, their epidemiological analysis has not been adequately and thoroughly described. Therefore, most oral and oropharyngeal cancer reports lack specificity and describe a general area of oral and pharyngeal issues, and diagnoses are also unclear. Owning to the biological and anatomical character of oral and oropharyngeal cancers and the specific criteria, diagnosis may not always be clear to a general practitioner. Dentists and dental specialists are the common referring clinicians for oral cancer patients. Every patient diagnosed with oral cancer needs a unique treatment plan, which proves to be a challenging, complex, and multidisciplinary problem for clinicians due to the solution's impact on their survival and quality of life (7).
Evidence suggests that males are more likely than females to be diagnosed with oral and oropharyngeal cancer, probably due to higher exposure to risk factors (8). The most important well-known risk factors for oral and oropharyngeal cancer are long-term and excessive alcohol consumption and tobacco use (9). Alcohol acts synergistically with tobacco, increasing the risk of oral and oropharyngeal cancer (10). The effect of different factors on the incidence of this cancer, such as gender, age, occupational, and geographical differences, affects its prevalence and causes epidemiological discrepancies (11, 12).
Due to the lack of screening programs and public knowledge in Iran, most patients are diagnosed with an advanced stage of oral cancer. This makes the burden of oral and oropharyngeal cancers very high, with a high economic cost (4). Consequently, planning a screening and early detection program for oral cancer may potentially decrease healthcare costs, morbidity, and mortality (13).
2. Objectives
Despite the importance of cancer studies, particularly oral and oropharyngeal cancer, due to inconsistencies in available incidence and prevalence data, etiology, and risk factors, only a few comprehensive and proper studies have been conducted in Iran (14). Therefore, the current study has been designed to investigate epidemiological features (incidence, ASR, the ratio of men to women, their types and locations, trends, demographics, and other specifications) of oral and oropharyngeal cancer in the Khuzestan province of Iran. We hope its information can benefit plans and interventions by clarifying available data in this field.
3. Methods
3.1. Study Design and Setting and Data Gathering
This cross-sectional study was conducted on raw data on cancer incidence, which was obtained from the Khuzestan cancer registry as a part of the Iranian population-based Cancer Registry from 2014 to 2019 after conformation by the Ahvaz Jundishapur University of Medical Sciences (AJUMS) ethics committee. The population and demographic data of the Khuzestan province during these five years were acquired from the official reports of the Statistical Centre of Iran (SCI).
3.2. Data Standardization and Processing
The recorded cases were standardized by ICD-10 and ICD-O-3 classifications and categorized by gender, age, age group, diagnosis, diagnosis site, diagnosis year, diagnosis method, and residency location (Khuzestan province counties).
Diagnosis sites were categorized according to the ICD-10 (International Classification of Diseases 10th Revision), and new cases for the oral cavity and upper aerodigestive tract (malignant neoplasms of lip, oral cavity, and pharynx C00-C14 in addition to the nasal cavity and middle ear, accessory sinuses, and larynx C30-C32) were extracted.
Histological subtypes were defined according to the International Classification of Diseases for Oncology, Third Edition (ICD-O-3).
The diagnosis methods in this study were pathologic reports, cytologic reports, clinical reports, and death certificates.
Age groups were established in standardized age-specific spans (E.g., 0 - 4, 5 - 9, 10 - 14, etc.) to make age-specific rate (ASR) and crude rate (CR) calculations possible
wi = world standard population; ri = number of incidence cases; ni = Person-years of observation
Ei = number of incidence cases; Pi = Mid-year mean population
3.3. Ethical Considerations
This study was part of a dissertation by Mr. Alireza Rahmanian Koushkaki at the School of Dentistry. In review, Raw data on cancer incidence was obtained from the Khuzestan cancer registry after being approved by the Ahvaz Jundishapur University of Medical Sciences (AJUMS) ethics committee (IR.AJUMS.REC.1398.855), (CRC-9821).
3.4. Statistical Analysis
Descriptive data for the frequency and rate of each variable and analyses of associations between variables were conducted employing the chi-squared test in SPSS 16.0 (SPSS Inc., Chicago, IL, USA) with the level of statistical significance P < 0.05 as the main statistical processing program.
4. Results
A total of 941 cases were identified during the study period, with the composition of males 675 cases and females 266 cases. The frequency of cases and distribution in each year is tabulated in Table 1.
| Age Groups | Male | Female | Total | Asr |
|---|---|---|---|---|
| Total | 7.77 | |||
| 0 - 4 | 2 (0.3) | 2 (0.8) | 4 (0.4) | |
| 5 - 9 | 2 (0.3) | 2 (0.8) | 4 (0.4) | |
| 10 - 14 | 5 (0.7) | 3 (1.1) | 8 (0.9) | |
| 15 - 19 | 8 (1.2) | 1 (0.4) | 9 (1) | |
| 20 - 24 | 7 (1) | 4 (1.5) | 11 (1.2) | |
| 25 - 29 | 12 (1.8) | 6 (2.3) | 18 (1.9) | |
| 30 - 34 | 19 (2.8) | 18 (6.8) | 37 (3.9) | |
| 35 - 39 | 26 (3.9) | 19 (7.1) | 45 (4.8) | |
| 40 - 44 | 50 (7.4) | 22 (8.3) | 72 (7.7) | |
| 45 - 49 | 76 (11.3) | 22 (8.3) | 98 (10.4) | |
| 50 - 54 | 63 (9.3) | 28 (10.5) | 91 (9.7) | |
| 55 - 59 | 78 (11.6) | 26 (9.8) | 104 (11.1) | |
| 60 - 64 | 96 (14.2) | 29 (10.9) | 125 (13.3) | |
| 65 - 69 | 61 (9) | 29 (10.9) | 90 (9.6) | |
| 70 - 74 | 52 (7.7) | 23 (8.6) | 75 (8) | |
| 75 - 79 | 47 (7) | 17 (6.4) | 64 (6.8) | |
| 80 - 84 | 46 (6.8) | 7 (2.6) | 53 (5.6) | |
| 85+ | 25 (3.7) | 8 (3) | 33 (3.5) | |
| Crude rate | 28.14 | 11.34 | 19.9 | |
| 2018 | 8.83 | |||
| 0 - 4 | 0 (0) | 0 (0) | 0 (0) | |
| 5 - 9 | 0 (0) | 1 (1.6) | 1 (0.5) | |
| 10 - 14 | 1 (0.6) | 0 (0) | 1 (0.5) | |
| 15 - 19 | 0 (0) | 1 (1.6) | 1 (0.5) | |
| 20 - 24 | 3 (1.9) | 1 (1.6) | 4 (1.8) | |
| 25 - 29 | 0 (0) | 3 (4.7) | 3 (1.4) | |
| 30 - 34 | 3 (1.9) | 6 (9.4) | 9 (4.1) | |
| 35 - 39 | 2 (1.3) | 3 (4.7) | 5 (2.3) | |
| 40 - 44 | 11 (7.1) | 6 (9.4) | 17 (7.8) | |
| 45 - 49 | 18 (11.6) | 3 (4.7) | 21 (9.6) | |
| 50 - 54 | 20 (12.9) | 8 (12.5) | 28 (12.8) | |
| 55 - 59 | 22 (14.2) | 5 (7.8) | 27 (12.3) | |
| 60 - 64 | 21 (13.5) | 8 (12.5) | 29 (13.2) | |
| 65 - 69 | 15 (9.7) | 5 (7.8) | 20 (9.1) | |
| 70 - 74 | 13 (8.4) | 6 (9.4) | 19 (8.7) | |
| 75 - 79 | 12 (7.7) | 4 (6.3) | 16 (7.3) | |
| 80 - 84 | 8 (5.2) | 1 (1.6) | 9 (4.1) | |
| 85+ | 6 (3.9) | 3 (4.7) | 9 (4.1) | |
| Crude rate | 6.4 | 2.72 | 4.59 | |
| 2017 | 8.34 | |||
| 0 - 4 | 0 (0) | 0 (0) | 0 (0) | |
| 5 - 9 | 1 (0.7) | 0 (0) | 1 (0.5) | |
| 10 - 14 | 1 (0.7) | 2 (3.4) | 3 (1.5) | |
| 15 - 19 | 3 (2.2) | 0 (0) | 3 (1.5) | |
| 20 - 24 | 1 (0.7) | 0 (0) | 1 (0.5) | |
| 25 - 29 | 4 (2.9) | 0 (0) | 4 (2) | |
| 30 - 34 | 4 (2.9) | 7 (11.9) | 11 (5.6) | |
| 35 - 39 | 6 (4.3) | 4 (6.8) | 10 (5.1) | |
| 40 - 44 | 8 (5.8) | 4 (6.8) | 12 (6.1) | |
| 45 - 49 | 22 (15.9) | 4 (6.8) | 26 (13.2) | |
| 50 - 54 | 6 (4.3) | 6 (10.2) | 12 (6.1) | |
| 55 - 59 | 19 (13.8) | 8 (13.6) | 27 (13.7) | |
| 60 - 64 | 20 (14.5) | 5 (8.5) | 25 (12.7) | |
| 65 - 69 | 12 (8.7) | 7 (11.9) | 19 (9.6) | |
| 70 - 74 | 9 (6.5) | 8 (13.6) | 17 (8.6) | |
| 75 - 79 | 6 (4.3) | 2 (3.4) | 8 (4.1) | |
| 80 - 84 | 10 (7.2) | 0 (0) | 10 (5.1) | |
| 85+ | 6 (4.3) | 2 (3.4) | 8 (4.1) | |
| Crude rate | 5.73 | 2.52 | 4.15 | |
| 2016 | 6.70 | |||
| 0 - 4 | 0 (0) | 0 (0) | 0 (0) | |
| 5 - 9 | 0 (0) | 0 (0) | 0 (0) | |
| 10 - 14 | 0 (0) | 0 (0) | 0 (0) | |
| 15 - 19 | 1 (0.8) | 0 (0) | 1 (0.6) | |
| 20 - 24 | 0 (0) | 0 (0) | 0 (0) | |
| 25 - 29 | 3 (2.3) | 0 (0) | 3 (1.7) | |
| 30 - 34 | 3 (2.3) | 1 (2.2) | 4 (2.3) | |
| 35 - 39 | 4 (3.1) | 3 (6.7) | 7 (4) | |
| 40 - 44 | 13 (9.9) | 6 (13.3) | 19 (10.8) | |
| 45 - 49 | 14 (10.7) | 5 (11.1) | 19 (10.8) | |
| 50 - 54 | 12 (9.2) | 4 (8.9) | 16 (9.1) | |
| 55 - 59 | 14 (10.7) | 5 (11.1) | 19 (10.8) | |
| 60 - 64 | 14 (10.7) | 5 (11.1) | 19 (10.8) | |
| 65 - 69 | 14 (10.7) | 8 (17.8) | 22 (12.5) | |
| 70 - 74 | 6 (4.6) | 2 (4.4) | 8 (4.5) | |
| 75 - 79 | 14 (10.7) | 5 (11.1) | 19 (10.8) | |
| 80 - 84 | 13 (9.9) | 1 (2.2) | 14 (8) | |
| 85+ | 6 (4.6) | 0 (0) | 6 (3.4) | |
| Crude rate | 5.48 | 1.94 | 3.74 | |
| 2015 | 8.04 | |||
| 0 - 4 | 1 (0.8) | 0 (0) | 1 (0.5) | |
| 5 - 9 | 1 (0.8) | 0 (0) | 1 (0.5) | |
| 10 - 14 | 2 (1.6) | 1 (1.8) | 3 (1.6) | |
| 15 - 19 | 3 (2.3) | 0 (0) | 3 (1.6) | |
| 20 - 24 | 1 (0.8) | 2 (3.6) | 3 (1.6) | |
| 25 - 29 | 1 (0.8) | 3 (5.4) | 4 (2.2) | |
| 30 - 34 | 6 (4.7) | 3 (5.4) | 9 (4.9) | |
| 35 - 39 | 8 (6.2) | 4 (7.1) | 12 (6.5) | |
| 40 - 44 | 8 (6.2) | 2 (3.6) | 10 (5.4) | |
| 45 - 49 | 12 (9.3) | 6 (10.7) | 18 (9.7) | |
| 50 - 54 | 16 (12.4) | 5 (8.9) | 21 (11.4) | |
| 55 - 59 | 10 (7.8) | 7 (12.5) | 17 (9.2) | |
| 60 - 64 | 22 (17.1) | 5 (8.9) | 27 (14.6) | |
| 65 - 69 | 12 (9.3) | 6 (10.7) | 18 (9.7) | |
| 70 - 74 | 7 (5.4) | 6 (10.7) | 13 (7) | |
| 75 - 79 | 9 (7) | 0 (0) | 9 (4.9) | |
| 80 - 84 | 6 (4.7) | 4 (7.1) | 10 (5.4) | |
| 85+ | 4 (3.1) | 2 (3.6) | 6 (3.2) | |
| Crude rate | 5.36 | 2.35 | 3.86 | |
| 2014 | 6.92 | |||
| 0 - 4 | 1 (0.8) | 2 (4.8) | 3 (1.8) | |
| 5 - 9 | 0 (0) | 1 (2.4) | 1 (0.6) | |
| 10 - 14 | 1 (0.8) | 0 (0) | 1 (0.6) | |
| 15 - 19 | 1 (0.8) | 0 (0) | 1 (0.6) | |
| 20 - 24 | 2 (1.6) | 1 (2.4) | 3 (1.8) | |
| 25 - 29 | 4 (3.3) | 0 (0) | 4 (2.4) | |
| 30 - 34 | 3 (2.5) | 1 (2.4) | 4 (2.4) | |
| 35 - 39 | 6 (4.9) | 5 (11.9) | 11 (6.7) | |
| 40 - 44 | 10 (8.2) | 4 (9.5) | 14 (8.5) | |
| 45 - 49 | 10 (8.2) | 4 (9.5) | 14 (8.5) | |
| 50 - 54 | 9 (7.4) | 5 (11.9) | 14 (8.5) | |
| 55 - 59 | 13 (10.7) | 1 (2.4) | 14 (8.5) | |
| 60 - 64 | 19 (15.6) | 6 (14.3) | 25 (15.2) | |
| 65 - 69 | 8 (6.6) | 3 (7.1) | 11 (6.7) | |
| 70 - 74 | 17 (13.9) | 1 (2.4) | 18 (11) | |
| 75 - 79 | 6 (4.9) | 6 (14.3) | 12 (7.3) | |
| 80 - 84 | 9 (7.4) | 1 (2.4) | 10 (6.1) | |
| 85+ | 3 (2.5) | 1 (2.4) | 4 (2.4) | |
| Crude rate | 5.13 | 1.79 | 3.47 |
A total of 941 cases were identified during the study period, with the composition of males 675 cases and females 266 cases. The frequency of cases and distribution in each year is tabulated in Table 1.
Cases were categorized into 18 age groups (0 - 4, 5 - 9, …, 80 - 84, 85+), demonstrating more than 75% of cases were from 40-79 years old, with the highest incidence was in the 60 - 64 age group with 13.3% of all cases. Even though the data showed a plateau trend in the age range of 60 - 69, an increasing trend is visible in the 50 - 59 years range to the point that in 2018, the range above comprised more than 25% of all cases in that year (Table 1).
Reported data in each year was acquired from four sources: Pathologic reports, cytologic reports, clinical reports, and death certificates. More than 85% of reported cases were submitted to the database with pathologic reports, which verifies high data validity (detailed data regarding other diagnosis methods and pathologic reports are visible in Table 2.
| Diagnosis Method and Year | Male | Female | Total | |||
|---|---|---|---|---|---|---|
| No. (%) | % in Row | No. (%) | % in Row | No. (%) | % in Row | |
| Pathology | ||||||
| Total | 593 (100) | 63 | 234 (100) | 24.9 | 827 (100) | 87.9 |
| 2018 | 139 (23) | 63.5 | 56 (24) | 25.6 | 195 (24) | 89 |
| 2017 | 123 (21) | 62.4 | 53 (23) | 26.9 | 176 (21) | 89.3 |
| 2016 | 119 (20) | 67.6 | 43 (18) | 24.4 | 162 (20) | 92 |
| 2015 | 110 (19) | 59.5 | 44 (19) | 23.8 | 154 (19) | 83.2 |
| 2014 | 102 (17) | 62.2 | 38 (16) | 23.2 | 140 (17) | 85.4 |
| Clinical | ||||||
| Total | 51 (100) | 5.4 | 12 (100) | 1.3 | 63 (100) | 6.7 |
| 2018 | 10 (20) | 4.6 | 3 (25) | 1.4 | 13 (21) | 5.9 |
| 2017 | 10 (20) | 5.1 | 3 (25) | 1.5 | 13 (21) | 6.6 |
| 2016 | 7 (14) | 4 | 1 (8) | 0.6 | 8 (13) | 4.5 |
| 2015 | 11 (22) | 5.9 | 3 (25) | 1.6 | 14 (22) | 7.6 |
| 2014 | 13 (25) | 7.9 | 2 (17) | 1.2 | 15 (24) | 9.1 |
| Cytology | ||||||
| Total | 1 (100) | 0.1 | 2 (100) | 0.2 | 3 (100) | 0.3 |
| 2018 | 0 (0) | 0 | 1 (50) | 0.5 | 1 (33) | 0.5 |
| 2017 | 0 (0) | 0 | 1 (50) | 0.5 | 1 (33) | 0.5 |
| 2016 | 0 (0) | 0 | 0 (0) | 0 | 0 (0) | 0 |
| 2015 | 1 (100) | 0.5 | 0 (0) | 0 | 1 (33) | 0.5 |
| 2014 | 0 (0) | 0 | 0 (0) | 0 | 0 (0) | 0 |
| Death certificate | ||||||
| Total | 30 (100) | 3.2 | 18 (100) | 1.9 | 48 (100) | 5.1 |
| 2018 | 6 (20) | 2.7 | 4 (22) | 1.8 | 10 (21) | 4.6 |
| 2017 | 5 (17) | 2.5 | 2 (11) | 1 | 7 (15) | 3.6 |
| 2016 | 5 (17) | 2.8 | 1 (6) | 0.6 | 6 (13) | 3.4 |
| 2015 | 7 (23) | 3.8 | 9 (50) | 4.9 | 16 (33) | 8.6 |
| 2014 | 7 (23) | 4.3 | 2 (11) | 1.2 | 9 (19) | 5.5 |
| Total | ||||||
| Total | 675 (100) | 71.7 | 266 (100) | 28.3 | 941 (100) | 100 |
| 2018 | 155 (23) | 70.8 | 64 (24) | 29.2 | 219 (23) | 100 |
| 2017 | 138 (20) | 70.1 | 59 (22) | 29.9 | 197 (21) | 100 |
| 2016 | 131 (19) | 74.4 | 45 (17) | 25.6 | 176 (19) | 100 |
| 2015 | 129 (19) | 69.7 | 56 (21) | 30.3 | 185 (20) | 100 |
| 2014 | 122 (18) | 74.4 | 42 (16) | 25.6 | 164 (17) | 100 |
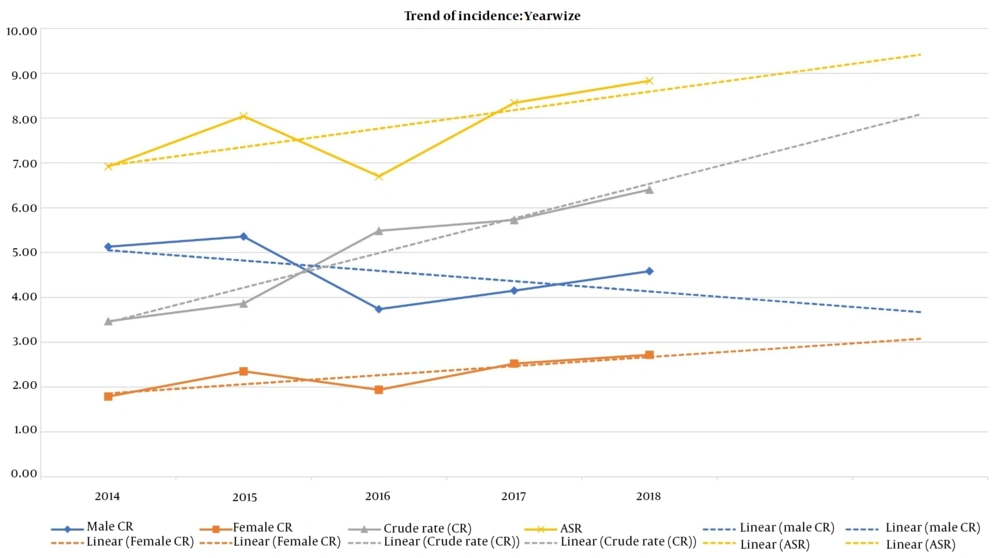
The number of reported cases each year increased compared to the year before, and the crude rate trend shows the same pattern as Figure 1 illustrates. The crude rate in female cases had an upward trend in contrast to the crude rate in males, which shows a downward trend. Detailed data regarding ASR and crude rates and trends are illustrated in Figure 1.
Tables 3 and 4 show diagnosis locations according to ICD-10 as mentioned in the methodology in each year and total. The most affected area code was C32 (larynx), and the second and third most affected areas were the oropharynx and tongue. Only 4 area codes account for 75% of all reported cases (C02, C07, C11, C32).
| Diagnosis Location | Age Groups | Total | P-Value | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 - 4 | 5 - 9 | 10 - 14 | 15 - 19 | 20 - 24 | 25 - 29 | 30 - 34 | 35 - 39 | 40 - 44 | 45 - 49 | 50 - 54 | 55 - 59 | 60 - 64 | 65 - 69 | 70 - 74 | 75 - 79 | 80 - 84 | 85+ | |||
| C00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 4 | 0.000 |
| C01 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | |
| C02 | 0 | 0 | 0 | 1 | 2 | 3 | 7 | 8 | 13 | 10 | 12 | 6 | 15 | 11 | 10 | 7 | 1 | 1 | 107 | |
| C03 | 0 | 1 | 0 | 0 | 1 | 1 | 4 | 2 | 2 | 2 | 5 | 3 | 3 | 4 | 1 | 1 | 1 | 1 | 32 | |
| C04 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 7 | |
| C05 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 3 | 0 | 2 | 5 | 2 | 0 | 0 | 0 | 2 | 19 | |
| C06 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 3 | 5 | 3 | 2 | 3 | 2 | 2 | 5 | 1 | 2 | 3 | 34 | |
| C07 | 0 | 1 | 1 | 2 | 0 | 5 | 5 | 6 | 4 | 4 | 5 | 4 | 5 | 11 | 3 | 1 | 3 | 3 | 63 | |
| C08 | 0 | 0 | 1 | 0 | 0 | 1 | 3 | 5 | 3 | 5 | 5 | 2 | 7 | 2 | 2 | 3 | 0 | 0 | 39 | |
| C09 | 0 | 0 | 0 | 1 | 0 | 2 | 1 | 0 | 1 | 1 | 3 | 2 | 2 | 3 | 0 | 3 | 0 | 1 | 20 | |
| C10 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 0 | 2 | 2 | 0 | 1 | 2 | 1 | 0 | 15 | |
| C11 | 1 | 0 | 5 | 3 | 4 | 2 | 9 | 9 | 9 | 14 | 11 | 22 | 17 | 5 | 8 | 3 | 4 | 0 | 126 | |
| C12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 5 | |
| C13 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 4 | 1 | 0 | 5 | 2 | 0 | 2 | 1 | 1 | 1 | 19 | |
| C14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2 | 2 | 0 | 0 | 1 | 1 | 8 | |
| C30 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 3 | 1 | 0 | 2 | 0 | 3 | 2 | 3 | 0 | 16 | |
| C31 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 1 | 2 | 0 | 3 | 0 | 2 | 2 | 0 | 2 | 0 | 0 | 16 | |
| C32 | 2 | 1 | 0 | 1 | 1 | 1 | 3 | 8 | 22 | 45 | 41 | 52 | 59 | 46 | 38 | 35 | 34 | 20 | 409 | |
| Total | 4 | 4 | 8 | 9 | 11 | 18 | 37 | 45 | 72 | 98 | 91 | 104 | 125 | 90 | 75 | 64 | 53 | 33 | 941 | |
a C00: Malignant neoplasm of lip, C01: Malignant neoplasm of the base of the tongue, C02: Malignant neoplasm of other and unspecified parts of the tongue, C03: Malignant neoplasm of gum, C04: Malignant neoplasm of floor of the mouth, C05: Malignant neoplasm of the palate, C06: Malignant neoplasm of other and unspecified parts of the mouth, C07: Malignant neoplasm of the parotid gland, C08: Malignant neoplasm of other and unspecified major salivary glands, C09: Malignant neoplasm of the tonsil, C10: Malignant neoplasm of the oropharynx, C11: Malignant neoplasm of the nasopharynx, C12: Malignant neoplasm of the pyriform sinus, C13: Malignant neoplasm of the hypopharynx, C14: Malignant neoplasm of other and ill-defined sites in the lip, oral cavity, and pharynx, C30: Malignant neoplasm of the nasal cavity and middle ear, C31: Malignant neoplasm of the accessory sinuses, C32: Malignant neoplasm of larynx.
| Diagnosis Pathology | Diagnosis Location | Total | P-Value | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C00 | C01 | C02 | C03 | C04 | C05 | C06 | C07 | C08 | C09 | C10 | C11 | C12 | C13 | C14 | C30 | C31 | C32 | |||
| Acinar cell carcinoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0.000 |
| Adenocarcinoma | 0 | 0 | 1 | 0 | 0 | 2 | 2 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 14 | |
| Adenoid cystic carcinoma | 0 | 0 | 0 | 1 | 1 | 3 | 0 | 12 | 9 | 1 | 0 | 3 | 0 | 0 | 1 | 1 | 2 | 0 | 34 | |
| Alveolar rhabdomyosarcoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | |
| Basal cell adenocarcinoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Basaloid carcinoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | |
| Basaloid SCC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 4 | |
| Burkitt lymphoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | |
| Carcinoma | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 8 | 6 | 0 | 0 | 73 | 0 | 1 | 0 | 1 | 0 | 13 | 103 | |
| Carcinoma in pleomorphic adenoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Carcinosarcoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | |
| Chordoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Embryonal rhabdomyosarcoma | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |
| Giant cell sarcoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Infiltrating duct carcinoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Leiomyosarcoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | |
| Lymphoepithelial carcinoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | |
| Malignant lymphoma | 0 | 0 | 1 | 2 | 0 | 1 | 1 | 1 | 4 | 12 | 5 | 11 | 0 | 1 | 1 | 3 | 3 | 3 | 49 | |
| Malignant myoepithelioma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Malignant Tumor | 1 | 1 | 6 | 6 | 1 | 2 | 6 | 4 | 6 | 2 | 2 | 18 | 0 | 2 | 3 | 1 | 1 | 64 | 126 | |
| Mantle cell lymphoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |
| Marginal zone B-cell lymphoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |
| Mature T-cell lymphoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | |
| Mucinous adenocarcinoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Mucoepidermoid carcinoma | 0 | 0 | 0 | 5 | 0 | 4 | 2 | 19 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 37 | |
| Olfactory neuroblastoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | |
| Plasmacytoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | |
| Pseudosarcomatous carcinoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | |
| Rhabdomyosarcoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | |
| SCC | 2 | 1 | 96 | 15 | 5 | 7 | 21 | 4 | 3 | 3 | 8 | 15 | 5 | 15 | 3 | 7 | 5 | 317 | 532 | |
| Verrucous carcinoma | 1 | 0 | 2 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 8 | |
| Total | 4 | 2 | 107 | 32 | 7 | 19 | 34 | 63 | 39 | 20 | 15 | 126 | 5 | 19 | 8 | 16 | 16 | 409 | 941 | |
a C00: Malignant neoplasm of lip, C01:Malignant neoplasm of the base of the tongue, C02: Malignant neoplasm of other and unspecified parts of the tongue, C03: Malignant neoplasm of gum, C04: Malignant neoplasm of floor of the mouth, C05: Malignant neoplasm of the palate, C06: Malignant neoplasm of other and unspecified parts of the mouth, C07: Malignant neoplasm of the parotid gland, C08: Malignant neoplasm of other and unspecified major salivary glands, C09: Malignant neoplasm of the tonsil, C10: Malignant neoplasm of the oropharynx, C11: Malignant neoplasm of the nasopharynx, C12: Malignant neoplasm of pyriform sinus, C13: Malignant neoplasm of the hypopharynx, C14: Malignant neoplasm of other and ill-defined sites in the lip, oral cavity, and pharynx, C30: Malignant neoplasm of the nasal cavity and middle ear, C31: Malignant neoplasm of accessory sinuses, C32: Malignant neoplasm of larynx.
As shown in Tables 3, and 4, association analysis between age groups, diagnosis locations, and diagnosis pathologies was statistically significant. Squamous cell carcinoma was the most frequently diagnosed malignancy, with 532 cases, accounting for more than 55% of all cases. Other reported diagnoses were acinar cell carcinoma, adenocarcinoma, adenoid cystic carcinoma, alveolar rhabdomyosarcoma, basal cell adenocarcinoma, basaloid carcinoma, basaloid SCC, Burkitt lymphoma, carcinoma, carcinoma in pleomorphic adenoma, carcinosarcoma, chordoma, embryonal rhabdomyosarcoma, giant cell sarcoma, infiltrating duct carcinoma, leiomyosarcoma, lymphoepithelial carcinoma, malignant lymphoma, malignant myoepithelioma, malignant tumors, mantle cell lymphoma, marginal zone B-cell lymphoma, mature T-cell lymphoma, mucinous adenocarcinoma, mucoepidermoid carcinoma, olfactory neuroblastoma, plasmacytoma, pseudosarcomatous carcinoma, rhabdomyosarcoma, and verrucous carcinoma (Table 4). Most cases in C32 area code were 40 years old and above. Most affected age groups in the C11 area were 45 - 65, with more than 50% of cases in C11. The third most prevalent affected area was C02; more than 70% of all cases affected were in the 40 - 75 age range (Table 3). As Table 4 shows, most cases in the C02 and C32 areas were diagnosed with SCC, and the most diagnosed pathology in the C11 area was carcinoma. Almost half of the cases affected in the C07 area were diagnosed with adenoid cystic carcinoma and mucoepidermoid carcinoma.
5. Discussion
Oral and oropharyngeal cancers are complex and multi-factorial, with a low incidence rate and an increasing trend, especially in younger age groups. This study categorized this disease into identifiable, standardized, and verifiable groups to address the emerging underlying correlations and patterns. The reports demonstrated a consistently increasing pattern in total cases, with a slight decrease in men and an increase in women. The increasing incidence and trend are a global phenomenon reflected in our reports. Still, contrary to the findings of Fu et al., trends among men were decreasing in this study. Women demonstrated an increasing trend, which was also evident in the study by Alshehri in Saudi Arabia, as well as other studies in Korea and Germany (13, 15-17). Age group assessment demonstrated that ages 40 - 69 accounted for more than 60% of all cases, with an inconsistent yet increasing trend among younger ages (40 - 54). Studies by Yamamoto and Shibahara and others confirm our pattern of incidence in the 50 - 69 age group as the most prevalent (18-20) and also the increasing rate of incidence among the under-50 population, as seen and forecasted finding in the study conducted by Hussein et al. and other regional and global studies (15, 21, 22).
The reported ASR in Table 1 shows a fluctuation throughout the study. Alshehri reported the same fluctuations in the Saudi Arabian population (16). However, contrary to the study above, our study's overall trend of ASR had an upward trajectory, illustrated in Figure 1.
Oral and oropharyngeal cancer trends appear to decline in the most studied male population. In contrast, the rising incidence was commonly observed among females, confirming the global pattern as Miranda-Filho and Bray also found in their global data analysis (11).
Further data analysis showed a statistical significance in the correlation between age groups and type and location of reported cancers and cancer sites and types. The most frequently reported cancer type was SCC in total, and most cancer sites were the tongue (C02), mouth (C06), and larynx (C32). Reporting SCC as the most prevalent cancer of the oral and oropharyngeal region has been confirmed in other studies, as Rabiei et al. have stated (18). The most affected cancer sites were C32, C11, C02 and C07. C32 areas and most frequently affected ages were 40 and above, with most cases in the 60 - 64 age group. Area C11 legions mostly affect ages 45 - 64. Other affected interludes in this area were 30 - 44 (demonstrated in Table 3), demonstrating that the younger population is mostly affected. Area C02 showed an interesting pattern. The affected range was 40 - 74, but the age group of 55 - 59 showed nearly half of the incidence compared to the mean number of cases in adjacent age interludes (40 - 54 and 60 - 74). Area C07 was mostly affected in the 65 - 69 age group, but the range of affected ages was wide and mostly visible from ages 25 to 69, once again affecting a younger array of cases. According to Table 4, some sites of interest appear among the most prevalent cancer types; specifically, for SCC, more than 75% of cases reported with squamous cell carcinoma were in the C02 and C32 sites. The reverse correlation was feasible as well. For example, in site C07, almost 50% of cases were diagnosed with mucoepidermoid and adenoid cystic carcinoma. These findings can assist in developing more precise and definitive guidelines for identifying oral and oropharyngeal cancers for specialists and the public. These findings regarding the nature of oral and oropharyngeal cancers as complex diseases with a high rate of mortality and morbidity (23) in oral diseases should promote robust and effective screening and management programs. Although the cost-effectiveness of oral screening programs in developing counties is still under debate (24), considering the role of public relations and informative tools and policies in synergy with consistent screening programs to eliminate risk factors needs to be accepted and be effective in an array of situations (13, 25, 26). Lifestyle choices have proven to play an important role in managing cancer vis-à-vis the importance of public relations and informative campaigns.
Tobacco use is identified as the most important yet avoidable risk factor for cancer, as it accounts for millions of annual cancer deaths (10). The malignancies caused by smoking include cancers of the lung, oral cavity, pharynx, larynx, esophagus, urinary bladder, renal, pelvis, and pancreas (27). Alcohol has also been considered carcinogenic to humans, causing oral cavity, pharynx, larynx, esophagus, and liver tumors. However, according to animal studies, ethanol has not been proven to be carcinogenic (28). Few studies managed to study nonsmoker alcohol users and smoker patients who did not drink (28). In one instance, the study of alcohol as an independent risk factor for oral leukoplakia was established in an Indian population (29). However, other studies evaluating the occurrence of oral epithelial dysplasia in nonsmoker drinkers found that alcohol's role in the development of oral epithelial dysplasia was influential solely in conjunction with tobacco (30). Several epidemiological and laboratory studies have established the relationship between diet and nutrition and the risk of cancer (31), as IARC affirms that a low intake of fruits and vegetables increases the risk of cancer (32). Other risk factors, namely occupational and environmental causes, such as exposure to solar radiation and ultraviolet (UV), sulfur dioxide, asbestos, pesticide exposures, mists from strong inorganic acids and the burning of fossil fuels, manufacturing of rubber products, plumbing (exposure to metals), woodworking and the automobile industry are also linked to oral and oropharyngeal cancers (33, 34) which are all active and present risk factors in Khuzestan. Due to the multi-factorial nature of oral and oropharyngeal cancers and their risk factors in Khuzestan, identifying, limiting, and eliminating these factors should be a priority.
The management of oral and oropharyngeal cancers is complex. It should be considered that management of all oral and oropharyngeal cancers should be conducted in a multidisciplinary manner due to the aesthetic and functional needs (breathing, speech, deglutition, sight, smell, taste, chewing, and jaw function) and potential critical temporary or permanent impairments by the tumor and its treatment (6). Due to facial and dental aesthetics' importance on social engagement, the tumour itself and its treatment may severely affect the self-esteem, confidence and social participation of oral cancer patients. In the management of oral and oropharyngeal cancer, dentists play a critical role, from the detection of premalignant lesions, early detection of oral cancers, management of oral cancers, patient’s dentition both before and post definitive treatment, surveillance of recurrent or new primary tumors in conjunction with the treating specialist, and rehabilitation of lost teeth in collaboration with the treating maxillofacial surgeon and prosthodontist (7). Thus, dental specialists should be more involved in screening programs, and their further involvement in designing support and rehabilitation regimens for the post-COVID era is crucial. According to the study by Dalanon and Matsuka, COVID-19 decreased interest regarding oral and oropharyngeal cancers on a global scale, especially in middle and low-income countries (35). In conjunction with the increasing incidence of oral and oropharyngeal cancers, particularly in younger age groups and females in this study and forecasted trends by GCO, emphasize the necessity for prioritizing cancers, particularly oral and oropharyngeal cancers, in Khuzestan.
5.1. Conclusions
The data on oral and oropharyngeal cancer in Khuzestan demonstrated that the overall incidence is increasing, particularly in women and younger age groups in the study period. The numbers in the ASR showed some fluctuations, but overall, the trend of the ASR continued to rise. Concerning increasing trends in this study and projections of GCO and the presence of the majority of oral and oropharyngeal cancer in the Khuzestan province of Iran, extensive screening and prevention programs in conjunction with rehabilitation programs should be on the agenda for policymakers to ensure high-risk populations (40 and above) have enough facilities and means to free themselves from this burden, and efforts are made to support the affected groups free of charge.
5.2. Limitations
Due to limitations in the acquired data from the Khuzestan cancer registry, this study has certain limitations. These limitations include discrepancies in data sets, missing data points, and a lack of verified data after 2019.