1. Background
End-stage renal disease (ESRD), defined as renal dysfunction, is rising worldwide, increasing by about 8% annually (1, 2). Hemodialysis is the most common treatment for this disease, which can cause many physical and mental problems in the long run (3). Sleep disorders are among the most common complaints in hemodialysis patients (4). The reported prevalence of sleep disorders in ESRD patients is 40 - 80% (5). A study on chronic hemodialysis showed that 87% of patients had poor sleep quality (6). Sleep disorders include delayed sleep, sleep apnea, restless legs syndrome (RLS), and periodic limb movement disorder (PLMD), which can lead to fatigue, restlessness, anxiety, depression, physical disability, and decreased quality of life (7-10). Benzodiazepines, which are used as first-line drugs in treating sleep disorders (11), have side effects such as drug tolerance, poor sleep quality, and exacerbation of sleep disorders (12).
End-stage renal disease can also affect patients’ mental and physical well-being (13, 14). A study found that spiritual well-being was negatively associated with depression, anxiety, and stress in hemodialysis patients (15). Another study among hemodialysis patients revealed a significant relationship between well-being and sleep quality (16). Lavender is a plant belonging to the mint genus whose leaves and green parts contain volatile essential oils. Lavender can be used as an essential oil, cream, gel, and lipogel. Numerous studies have examined the effects of lavender on anxiety, depression, stress, fatigue, and pain in patients undergoing hemodialysis and have reported positive results (7, 17, 18). Several studies have shown that aromatherapy with lavender essential oil can improve the sleep quality of patients undergoing maintenance hemodialysis (19, 20). However, a study reported that aromatherapy with lavender essential oil had no significant effect on sleep quality in patients undergoing maintenance hemodialysis (21). Another study revealed that aromatherapy with lavender essential oil in concentrations of 40 - 50% could positively affect the well-being of patients undergoing maintenance hemodialysis (22). Our review found inconclusive results on lavender aromatherapy's effect on hemodialysis patients' sleep quality and well-being. Therefore, our research team decided to change the lavender route of administration. Lipogel is a lipid-based gel that inhibits the defense biofilm and has several advantages, such as its simple manufacturing process and long-term thermodynamic stability (23, 24). Considering the soothing effect of massage rather than aromatherapy (25), we used a lipogel form of lavender to address the aim of the present study.
2. Objectives
The present study aimed to assess the effect of topical application of lipogel containing lavender essential oil on hemodialysis patients' sleep quality and well-being.
3. Methods
3.1. Study Design
The present double-blinded clinical trial study was conducted at the dialysis center of Mazandaran University of Medical Sciences, Sari, Iran. The center has 96 dialysis machines that service patients undergoing hemodialysis in three shifts.
3.2. Sampling
The hemodialysis patients were enrolled in the study based on the inclusion criteria to evaluate the therapeutic effects of topical application of lavender lipogel on sleep quality and well-being in patients undergoing maintenance hemodialysis. Based on a study by Diggle et al. (26), the sample size was calculated as 40 individuals in each group, assuming a significance level of less than 5%, power of 80%, and effect size of 0.50. Initially, the participants were selected via the convenience sampling method. Then, they were randomly assigned to the experimental, control, and placebo groups via permuted block randomization. The statistical researcher assigned all patients to 20 blocks, 6 people in each block using a sealed envelope (non-transparent), and then allocated them to three groups based on random sequencing. Sample size formula:
The inclusion criteria were the age of 18 years and older, ESRD diagnosis, regular dialysis treatment at least 3 times a week for 6 months, ability to understand, read, and write Persian, no history of drug or non-drug allergies, no history of mental disorders, no experience of any stressful events in the last 6 months, and no use of sedatives. The study exclusion criteria included absence from more than 3 sessions of lavender lipogel treatment, need for additional dialysis for any reason, the existence of any wounds, edema, and lesions in the lipogel application area (the patient’s feet), history of alcohol and drug addiction, and pregnancy and breastfeeding. The patients were excluded from the study because of lavender allergy, unwillingness to continue participating, and lack of access to a dialysis center due to changing residence. The occurrence of any allergy to lavender was also recorded based on self-report.
3.3. Procedure
Eligible patients were identified after obtaining the code of ethics and permission to start sampling in the dialysis center. The researcher provided the necessary explanations to the eligible patients and selected the participants after obtaining informed consent. Then, the demographic characteristics of the patients were recorded, and the Yield Well-being Scale and Pittsburgh sleep quality index (PSQI) were completed. The necessary explanations of the study process were provided to the patients, and an informed consent form was obtained from them. In the experimental group, while the patients were lying on the bed during hemodialysis, one of the researchers wore gloves and removed 2 grams of lipogel containing 5% lavender essential oil purchased from the Barij Essential Oil Company from its container. She applied the cream evenly on the patient's feet, measuring 5 cm from top to bottom and 2 fingers wide from ankle to toe for a minute (27).
In the placebo group, the same amount of liposol without lavender essential oil, in a container identical to those containing lipogel with essential oil in terms of packaging and content, was rubbed onto the patient’s feet in the same method. All three groups (experimental, placebo, and control) received routine care. Routine care consists of patient education and nursing care based on the patient’s demand, including nutritional education, control of vital signs, and increasing the patient’s knowledge. All interventions were performed by a single person, the first author, who was trained under the supervision of experts, during hemodialysis while the patient was lying on a bed. Before and after the 12 intervention sessions, patients' sleep quality and well-being were assessed using the PSQI and Yield Well-being Scale, respectively. All data were collected by a single evaluator (the third author) who was blinded to the random assignment of the patients in the groups.
The lavender essential oil was purchased from Barij Essence Pharmaceutical Company (Kashan, Iran). To approve the plant used in the essential oil preparation, a sample of each lavender herbarium was received from the company, and its type and species were examined. To standardize the essential oil of the drug and the stability of the essential oil in the drug, the amount of linalyl acetate was determined using gas chromatography (GC) in the laboratory of the Deputy for Food and Drug of Mazandaran University of Medical Sciences. The aforementioned essential oil was applied homogeneously at the rate of 5% in the lipogel plasty base. To prepare the lipogel plasty base, 5% linear low-density polyethylene (LLDPE) was dissolved in 95% liquid paraffin at 90°C and heated indirectly at 130°C for 2 - 3 hours. It was then cooled very quickly to form the lipogel plasty base. The lipogel obtained from the plasty base was evaluated for physical stability at ambient temperature in a refrigerator for turbidity, syneresis, apparent changes in consistency, and pH. Lipogel products containing 5% lavender extract were prepared, and the physical stability of these products was evaluated in terms of viscosity, transparency, and syneresis for up to 3 months at 4, 25, and 40°C. The product sample was optimal regarding physical stability, and other tests were used in the clinical trial. All lipogel tubes containing essential oil and placebo were prepared in 1 step and had an expiration date of up to 6 months.
3.4. Instruments
The data collection tools used included a socio-demographic information questionnaire (including age, sex, education level, marital status, occupation, place of residence, socioeconomic status, body mass index (BMI), and underlying diseases), Yield Well-being Scale, and the PSQI. The Yield Well-being Scale consists of 5 items and measures well-being over the past 4 weeks. The total score of this tool ranges between 5 and 15; the scores 12 - 15, 8 - 11, and 5 - 7 indicate good, moderate, and poor well-being, respectively. The psychometric properties of this scale had been investigated on the Iranian elderly for the first time (28). The validity of the Persian version of this tool has been reported as 0.81 by Cronbach's alpha method (29). The PSQI measures sleep quality over the past month. This index includes 19 items in the 7 sections of mental sleep quality, sleep delay, sleep duration, sleep adequacy, sleep disorder, sleep medication use, and daily dysfunction. The score of each section is within the range of 0 - 3. The total score of the index is within the range of 0 - 21, and higher scores indicate poorer sleep quality. A score above 5 indicates poor sleep quality (30). The validity and reliability of the Persian version of the PSQI have been reported as 0.83 (31) and 0.88 (32), respectively.
3.5. Ethical Considerations
The study protocol was approved by the Ethics Committee at the Biomedical Research of Mazandaran University of Medical Sciences (IR.MAZUMS.REC.1398.1156) and was registered on the Iranian Registry of Clinical Trials (IRCT20191007045019N1). The patients were provided with the necessary information on the study's objectives, its process, and the type of intervention. Moreover, they were assured that their information would remain confidential. The patients were free to participate in or leave the study at any stage. They were also assured that refusing to participate or leaving the study would not affect their treatment and care. The written informed consent form was obtained from all patients.
3.6. Data Analysis
The data were analyzed using IBM Corp.'s SPSS software, version 21, located in Armonk, NY, USA. Statistical tests were used to detect differences between the three groups. The Kolmogorov-Smirnov (KS) test was employed to investigate the normal distribution of variables. Analysis of variance (ANOVA) was utilized for quantitative variables (including age), and the chi-squared and Fisher's exact tests were utilized for qualitative variables (including gender, marital status, education level, place of residence, and occupation). To compare the mean score of well-being among three groups and intra-groups, Kruskal-Wallis and Friedman tests were used, respectively. Analysis of variance and the Kruskal-Wallis test were used to compare the total scores of PSQI and its subscales among the three groups. The intra-group comparison of the PSQI total score and its subscales were assessed using paired t-test and Wilcoxon, respectively. A P-value less than 0.05 was considered statistically significant.
4. Results
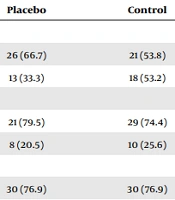
Of 145 hemodialysis patients, 25 did not meet the inclusion criteria. Then, 120 hemodialysis patients were randomly allocated to the three groups. Finally, the analysis was done on 113 patients due to sample attrition (experimental group: 4 patients for renal transplantation and one patient died, placebo group: One patient for renal transplantation, control group: One patient for renal transplantation). The mean ages of the patients in the experimental, placebo, and control groups were 58.43 ± 14.43, 63.36 ± 11.77, and 62.03 ± 12.59 years, respectively. Most of the patients were women (57.5%), had a pre-diploma degree (72.6%), were married (77.0%), were housewives (53.1%), and were urban residents (74.3%) (Table 1). The 3 groups (experimental, control, and placebo) did not differ significantly before the intervention regarding demographic variables.
| Variables | Groups | Significance Level and Statistical Test | ||
|---|---|---|---|---|
| Experimental | Placebo | Control | ||
| Gender | P = 0.353 b | |||
| Female | 18 (51.4) | 26 (66.7) | 21 (53.8) | |
| Male | 17 (48.6) | 13 (33.3) | 18 (53.2) | |
| Educational level | P = 0.265 b | |||
| Non-academic degree | 22 (62.9) | 21 (79.5) | 29 (74.4) | |
| Academic degree | 13 (37.1) | 8 (20.5) | 10 (25.6) | |
| Marital status | P > 0.999 b | |||
| Married | 27 (77.1) | 30 (76.9) | 30 (76.9) | |
| Single | 8 (22.9) | 9 (23.1) | 9 (23.1) | |
| Job | P = 0.759 b | |||
| Self-employed | 6 (17.1) | 10 (25.6) | 6 (15.4) | |
| Retired | 8 (22.9) | 5 (12.8) | 10 (25.6) | |
| Housewife | 18 (51.4) | 22 (56.4) | 20 (51.3) | |
| Other | 3 (8.6) | 2 (5.1) | 3 (7.7) | |
| Place of residence | P = 0.666 b | |||
| Urban | 27 (77.1) | 27 (69.2) | 30 (76.9) | |
| Rural | 8 (22.9) | 12 (30.8) | 9 (23.1) | |
| Economic status | P = 0.234 b | |||
| Low | 11 (31.4) | 13 (33.3) | 19 (48.7) | |
| Average and high | 24 (68.6) | 26 (66.7) | 20 (51.3) | |
| BMI | P = 0.398 b | |||
| Normal and low | 20 (57.1) | 15 (38.5) | 14 (35.9) | |
| Overweight | 8 (22.9) | 14 (35.9) | 14 (35.9) | |
| Obese | 7 (20.0) | 10 (25.6) | 11 (28.2) | |
| Age | 58.43 ± 14.43 | 63.36 ± 11.77 | 62.03 ± 12.59 | P = 0.247 c |
The Socio-demographic Characteristics of the Participants a
The mean (SD) of the well-being score in experimental, placebo, and control groups is presented in Table 2. The well-being score was 10.26 (2.43), 10.21 (2.82), and 9.62 (3.14) after implementation in the experimental, placebo, and control groups, respectively. The mean (SD) well-being score comparison showed a non-significant difference among the three groups before and after the study (P = 0.739 vs. P = 0.535).
The Mean ± SD of the Well-being Score in Experimental, Placebo, and Control Groups
The groups (experimental, control, and placebo) did not differ significantly before the intervention in terms of the total score of sleep quality (experimental group: 9.20 ± 3.66, control group: 9.64 ± 5.03, placebo group: 10.36 ± 4.49, P = 0.527). Still, a significant difference was observed after the intervention (experimental group: 6.94 ± 3.97, control group: 9.15 ± 4.20, placebo group: 9.51 ± 4.00, P = 0.017). Moreover, no significant change was observed in the control (9.64 ± 5.03 vs. 9.15 ± 4.20, P = 0.366) and placebo groups (10.36 ± 4.49 vs. 9.51 ± 4.00, P = 0.237) after the intervention; however, a significant change was observed in the experimental group after the intervention (9.20 ± 3.66 vs. 6.94 ± 3.97, P = 0.007) (Table 3).
| Sleep Quality | Groups | Significance Level and Statistical Test | ||
|---|---|---|---|---|
| Experimental | Placebo | Control | ||
| Subjective sleep quality (range of the subscale score 0 - 3) | ||||
| Before | 1.37 ± 0.73 | 1.51 ± 0.79 | 1.51 ± 0.97 | H = 0.438, P = 0.803 b |
| After | 1.09 ± 0.81 | 1.31 ± 0.76 | 1.36 ± 0.90 | H = 2.464, P = 0.292 b |
| Intra-group comparison | Z = -1.78, P = 0.070 a | Z = -1.62, P = 0.106 a | Z = -1.16, P = 0.243 a | - |
| Sleep latency (range of the subscale score 0 - 3) | ||||
| Before | 1.97 ± 0.95 | 1.92 ± 1.13 | 1.87 ± 1.12 | H = 0.056, P = 0.972 b |
| After | 1.49 ± 0.95 | 1.64 ± 1.13 | 1.72 ± 1.12 | H = 0.899, P = 0.638 b |
| Intra-group comparison | Z = -2.38, P = 0.017 a | Z = -1.55, P = 0.120 a | Z = -0.638, P = 0.520 a | - |
| Sleep duration (range of the subscale score 0 - 3) | ||||
| Before | 1.63 ± 1.26 | 1.97 ± 1.08 | 1.95 ± 1.07 | H = 1.880, P = 0.391 b |
| After | 1.29 ± 1.17 | 1.90 ± 0.96 | 1.77 ± 1.01 | H = 6.268, P = 0.044 b |
| Intra-group comparison | Z = -1.36, P = 0.170 a | Z = -1.504, P = 0.610 a | Z = - 1.34, P = 0.180 a | - |
| Sleep efficiency (range of the subscale score 0 - 3) | ||||
| Before | 1.14 ± 1.19 | 1.56 ± 1.33 | 1.44 ± 1.35 | H = 1.524, P = 0.467 b |
| After | 0.74 ± 0.95 | 1.33 ± 1.28 | 1.59 ± 1.22 | H = 0.828, P = 0.012 b |
| Intra-group comparison | Z = -1.61, P = 0.106 a | Z = -1.07, P = 0.283 a | Z = -0.71, P = 0.478 a | - |
| Sleep disturbance (range of the subscale score 0 - 3) | ||||
| Before | 1.34 ± 0.48 | 1.31 ± 0.46 | 1.21 ± 0.61 | H = 0.894, P = 0.640 b |
| After | 1.03 ± 0.45 | 1.26 ± 0.44 | 1.10 ± 0.44 | H = 2.739, P = 0.094 b |
| Intra-group comparison | Z = -2.84, P = 0.005 a | Z = -0.58, P = 0.560 a | Z = -1.15, P = 0.250 a | - |
| Use of medication for sleep (range of the subscale score 0 - 3) | ||||
| Before | 0.54 ± 1.03 | 0.82 ± 1.21 | 0.46 ± 0.94 | H = 2.627, P = 0.269 b |
| After | 0.46 ± 0.95 | 0.90 ± 1.23 | 0.51 ± 1.02 | H = 4.077, P = 0.130 b |
| Intra-group comparison | Z = -0.41, P = 0.680 a | Z = -0.75, P = 0.450 a | Z = -0.35, P = 0.720 a | - |
| Daytime dysfunction (range of the subscale score 0 - 3) | ||||
| Before | 1.25 ± 0.90 | 1.26 ± 0.96 | 1.21 ± 0.86 | H = 0.124, P = 0.940 b |
| After | 0.86 ± 0.81 | 1.18 ± 0.94 | 1.10 ± 0.88 | H = 2.636, P = 0.268 b |
| Intra-group comparison | Z = -1.59, P = 0.110 a | Z = -0.41, P = 0.680 a | Z = -0.59, P = 0.550 a | - |
| Total sleep quality score (range of the scale score 0 - 21) | ||||
| Before | 9.20 ± 3.66 | 10.36 ± 4.49 | 9.64 ± 5.03 | F = 0.641, P = 0.527 c |
| After | 6.94 ± 3.97 | 9.51 ± 4.00 | 9.15 ± 4.20 | F = 4.251, P = 0.017 c |
| Intra-group comparison | t = 2.869, P = 0.007 d | t = 1.20, P = 0.237 d | t = 0.915, P = 0.366 d | - |
Mean and Standard Deviation of Sleep Quality of Participants Before and After the Intervention
Also, the effect size of the total sleep quality score was 0.56, 0.21, and 0.11 in the experimental, placebo, and control groups, respectively.
5. Discussion
The current randomized, controlled clinical trial was conducted on hemodialysis patients to address the clinical question of whether the topical application of lipogel containing lavender essential oil can improve the sleep quality and well-being of hemodialysis patients. The findings showed that, although the mean and standard deviation of well-being increased in the intervention and placebo groups and decreased in the control group, this difference after the intervention among the 3 groups was not significant. In the experimental group, the total sleep quality, duration, and efficiency score decreased after the intervention.
The present study showed no statistically significant difference among the three groups regarding well-being. Bagheri-Nesami et al. investigated the effects of aromatherapy with lavender on the well-being of hemodialysis patients. They discovered that lavender essential oil at concentrations of 40% and 50% had a positive impact on well-being, while concentrations of 10 - 30% did not produce significant effects (22). The difference between the results of this study and the present study may be due to differences in the concentration of lavender essential oil, the drug form used, and the time of lavender use during each intervention session, which was 20 minutes in this study. Fatigue is one of the most important complaints of hemodialysis patients and affects their well-being and QOL (33). In another study, one-third of hemodialysis patients reported fatigue during the first hours of hemodialysis sessions, and a quarter reported severe fatigue after hemodialysis (34). Bagheri-Nesami et al. conducted a clinical trial to determine the effect of aromatherapy with lavender essential oil on the fatigue level of 59 hemodialysis patients. They found no significant difference between the fatigue scores of the 2 groups before the intervention and 2 and 4 weeks after it (35). Other factors affecting hemodialysis patients' well-being and QOL include anxiety and depression. In a clinical trial conducted by Bagheri-Nesami et al., the level of depression and anxiety in patients was assessed at the beginning and at the end of the second and fourth weeks in the first hour of the hemodialysis session to assess the effect of aromatherapy with lavender essential oil on these variables. The study's results did not show a significant difference between the 2 groups in terms of the severity of anxiety before the intervention and two and four weeks after it. Nevertheless, in the intervention group, the incidence of depression was significantly lower (36). The prevalence of anxiety among hemodialysis patients has been reported as 27 - 50% (37, 38). Stressors and lifestyle changes have had a negative effect on the well-being of these patients and their coping skills in the face of various stressors in life, including the process of hemodialysis treatment, which leads to a decrease in their QOL (39).
The results of the present study showed no significant difference among the groups in terms of the sleep quality score before the intervention. In the experimental group, the total sleep quality score decreased after the intervention, while in the other two groups, no significant changes were observed. In a study conducted by Najafi et al., there was no statistically significant difference in the average total score of sleep quality at the beginning of the study between the two groups. However, at the end of the study, a statistically significant difference was observed between the groups (40). Kasra Dehkordi et al. found no significant difference in the mean sleep quality score in the experimental and control groups not only before the intervention but also after using lavender essential oil (21). The results of this study were inconsistent with those of the present study. This could be due to differences in sample size and intervention design method.
The present study showed a significant difference in sleep quality and efficiency after the intervention. The Kruskal-Wallis H test results showed a significant difference between the intervention and placebo groups regarding sleep duration and between the intervention and control groups regarding sleep efficiency. A study was conducted by Dabirian et al. to determine the effect of inhalation of lavender essential oil on the sleep quality of 53 patients undergoing hemodialysis. The intervention consisted of inhalation of lavender essential oil from a cotton ball impregnated with 2 drops of essential oil at a distance of 15 - 20 cm from the patients' pillows 3 times a week before bedtime for four weeks. The results showed a statistically significant difference between the participants' mean score of total sleep quality before and after inhalation of lavender essential oil. The score of each dimension of sleep quality after inhalation was significantly lower than before inhaling the essential oil. In other words, inhaling lavender essential oil improved sleep quality, including subjective sleep quality, sleep latency, sleep adequacy, sleep duration, and daily sleep dysfunction in hemodialysis patients (19). This study's results align with those of the present study. In the study by Senturk and Tekinsoy Kartin, the effect of inhalation of lavender oil on the level of anxiety and sleep quality of hemodialysis patients was investigated. In this study, 2 drops of lavender essential oil were placed at a 15 - 20 cm distance from the patients' pillows half an hour before bedtime for a week. The study results showed that, in the intervention group, compared to the control group, subjective sleep quality was higher, and the average score of sleep duration increased. Still, the average score of drowsiness during the day decreased. In addition, the results showed that the difference in the mean score of sleep time between the two groups was not significant. Still, there was a significant difference between the intervention and control groups in the average total score of anxiety and anxiety scales (41).
5.1. Limitations
The study's limitation was assessing patients’ well-being and sleep quality through a self-report method, which could have caused some errors.
5.2. Conclusions
The present study's findings revealed that lipogel containing lavender essential oil is an effective method to improve the sleep quality of hemodialysis patients. It can be a cost-effective and easy method in hemodialysis centers. It is recommended to conduct additional studies to confirm the current findings.