1. Background
Demyelinated primary plaques are a symptom of the central nervous system disorder known as multiple sclerosis (MS). Young individuals are more likely to suffer from this chronic inflammatory illness, which makes them more likely to have psychological, social, and financial difficulties. However, despite the efforts made to elucidate the etiopathology of this disease, its understanding and treatment are still complex and challenging (1). Currently, MS affects more than 2.5 million individuals worldwide (2). The prevalence of MS has increased all over the world since 2013. According to the report of 75 countries, the incidence of MS is about 0/5 per 100,000 individuals per year (3). According to reports, Iran’s MS prevalence and incidence range from 5.3 to 89 per 100,000 individuals and 7 to 1.148 per 100,000 individuals, respectively (4). Multiple sclerosis is currently the second cause of disability among Iranian youth (5). Multiple sclerosis has been divided into several phenotypes by Lublin et al., including clinically isolated syndrome (CIS), primary-progressive MS (PPMS), secondary-progressive MS (SPMS), and relapsing-remitting MS (RRMS) (6, 7).
Unfortunately, psychological disorders, such as depression, in patients with MS hurt their quality of life (5). A person’s understanding of his/her place in life about goals, expectations, standards, and concerns and the culture and value systems in which individuals live are referred to as their quality of life (QoL). According to many studies, quality of life in patients with MS is associated with a worse prevalence of depression and fatigue than in healthy individuals (8-16).
Multiple sclerosis severely reduces the quality of life by reducing the ability to work and social activities. Current treatments for MS cannot completely cure the disease or reverse its progression. Additionally, patients need treatments that preserve abilities as much as possible, optimize function, and improve quality of life at all stages of disability. For some patients, even those receiving disease-controlling drugs, improving their quality of life remains an unmet need. Incorporating healthcare decisions, assessing the quality of life, and considering symptomatic treatments can address the important needs of patients with MS (17). Additionally, the patient’s quality of life varies based on social and demographic characteristics, such as socioeconomic class, degree of education, and marital status, all of which might exacerbate the illness (18). Generally, the impact of MS on the quality of life can be influenced by factors such as type of MS, education, age, job, social support, employment, and disability level (19-23).
On the other hand, findings indicate that anxiety and depression are common in MS (24, 25). Studies have shown that the risk of depression, stress, and anxiety is high in MS patients (26); however, according to the results of some studies, except in a few cases, anxiety showed a greater effect than depression. Although poor education and addiction were predictors of depression, anxiety was also connected with younger age and shorter duration of illness (27). Additionally, stress is a commonly reported concern of patients with MS (28). The results of Bastani et al.’s study showed that more than half of the women with MS studied had high perceived stress (29). Based on the results of Karimi et al.’s study, it was observed that the majority of the studied population, including patients with MS, had moderate levels of depression, anxiety, and stress (26).
Studies on the role of psychological factors show different findings. The findings of the studies indicate that anxiety plays an important role in individuals’ perception and health as much as depression, which subsequently affects the severity of symptoms and the overall quality of life. It seems that reducing the effects of depression and anxiety requires an early diagnosis of these problems and enhancing social support (27). Additionally, depression’s mood has a significant impact on one’s quality of life. The physical dimensions of quality of life are most affected by the condition of disability, fatigue, and poor sleep quality (12). Some studies indicate that depression has a greater impact on quality of life than anxiety (30); nevertheless, others consider anxiety to be a more influential factor (31). On the other hand, in most studies, one or two psychological variables have been discussed as predictors of quality of life. Meanwhile, in the present study, important psychological factors, such as anxiety, depression, and perceived stress, have been considered the main variables.
2. Objectives
Therefore, it seems that identifying factors related to the quality of life can help healthcare systems to adjust these factors and improve the quality of life of these patients, given the significance of improving the quality of life of patients with MS and the role of various factors based on studies. Therefore, the present study was conducted to determine the quality of life and its related factors in patients with MS.
3. Methods
3.1. Participants and Study Design
The present study was a cross-sectional descriptive-analytical study. The research environment was Guilan MS Association, Guilan, Iran, and the research community was all patients with MS referred to the Guilan MS Association between October 2021 and September 2022, who were included in the study by the convenience sampling method. Multiple linear regression analysis was utilized since the primary objective of the present research was to identify the variables that affect the quality of life in patients with MS. As a rule of thumb, in regression analyses for each independent variable (predictor), at least 15 individuals (in other sources, 20 or 10 subjects) should be selected (32). About 200 individuals were chosen since there are 12 potential prognostic factors in this research.
In addition, the inclusion criteria included at least one year of MS diagnosis and patients with an age range of 18 - 60 years. The exclusion criteria included a history of other underlying diseases and a lack of consent to participate in the study.
3.2. Ethical Considerations
Ethical approval for this study was obtained from the Ethics Committee of Guilan University of Medical Sciences, Rasht, Guilan, Iran (ethics code: IR.GUMS.REC.1400.377), and online consent was obtained from the patients.
3.3. Instruments
In this study, the questionnaire consists of 5 parts, namely demographic information, quality of life in MS patients by the Multiple Sclerosis Impact Scale (MSIS-29), Patient Health Questionnaire-9 (PHQ-9), Generalized Anxiety Disorder-7 (GAD-7), and Perceived Stress Scale-4 (PSS-4).
3.4. Demographic Variables
Demographic variables, including age, gender, marital status, education, occupation, place of residence, chronic disease, insurance, supplementary insurance, disease duration, and type of MS, were collected.
3.5. Perceived Stress Scale-4
The Perceived Stress Scale-4 (PSS-4) is a short form of the original PSS-14 item (PSS-14) that measures the perceived stress (33). Respondents rate items on a 5-point Likert scale, ranging from 0 (never) to 4 (very often). Total scores can range from 0 to 16, with high scores indicating a greater level of stress. The Persian version of this scale is reported to have satisfactory psychometric properties (34). In the present study, Cronbach’s alpha coefficient of the PSS-4 was 0.844.
3.6. Generalized Anxiety Disorder-7
The Generalized Anxiety Disorder-7 (GAD-7) is a 7-item self-report measure that assesses the presence of GAD symptoms over the past two weeks (35). Each item is rated on a 4-point Likert-type scale ranging from 0 (not at all) to 3 (nearly every day). Total scores can range from 0 to 21; higher scores indicate more GAD symptoms. A score of 10 or more is recommended as the reflection of a possible diagnosis of GAD. The GAD-7 has demonstrated sound psychometric properties in Iran (36). In the present study, Cronbach’s alpha coefficient of the GAD-7 was 0.905.
3.7. Patient Health Questionnaire-9
The Patient Health Questionnaire-9 (PHQ-9) is a 9-item self-report measure that assesses the presence of depressive symptoms over the past two weeks (37). Each item is rated on a 4-point Likert-type scale ranging from 0 (not at all) to 3 (nearly every day). Total scores can range from 0 to 27; higher scores indicate more depressive symptoms. A score of 10 or more is recommended as reflecting a possible diagnosis of depressive disorder. The PHQ-9 has demonstrated sound psychometric properties in Iran (38). In the current study, Cronbach’s alpha coefficient of the PHQ-9 was 0.908.
3.8. Multiple Sclerosis Impact Scale
The Multiple Sclerosis Impact Scale (MSIS-29) is a questionnaire on the quality of life in patients with MS by Hobart et al. in 2001 (39) to measure the quality of life. It was specially designed for patients with MS. This questionnaire contains 29 questions; the first 20 questions measure the physical impact, and the last 9 questions measure the psychological impact of MS on the patient. Each question has 5 options with a score of 1 to 5. The minimum possible score is 29, and the maximum is 145. Scores between 29 - 58, 58 - 87, and above 87 indicate low, average, and high quality of life, respectively. The Persian version of this scale is reported to have satisfactory psychometric properties (40). In the present study, Cronbach’s alpha coefficient of the physical and psychological subscales were 0.975 and 0.944, respectively.
3.9. Statistical Analysis
In this study, continuous variables were expressed as mean ± standard deviation (SD) and categorical variables as number (%). Pearson correlation coefficient was performed to investigate the relationship of MSIS-29 with PSS-4, GAD-7, and PHQ-9 scores. In univariable analysis, the relationships between demographic variables and MSIS-29 scores were investigated using independent samples t-test, one-way analysis of variance (ANOVA), and Pearson correlation coefficient. Then, hierarchical multiple linear regression was carried out to examine the relationship of 2 major factors with MSIS-29 scores: The demographic characteristics and measures of PSS-4, GAD-7, and PHQ-9. Two steps were conducted; accordingly, the demographic variables were entered in the first block, while the measures of PSS-4, GAD-7, and PHQ-9 were entered in the second block. The goodness of fit of the regression models was evaluated using coefficients of determination (R2). R2 is the proportion of variation in the dependent variable explained by the regression model. In addition, ∆R2, which is the change in R2 between the two models, was calculated. The models were also checked for multicollinearity through the variance inflation factor (VIF) and tolerance.
As a rule of thumb, a tolerance less than 0.2 and/or VIF more than 5 reflect a problem with multicollinearity. In this study, none of the variables showed significant multicollinearity. All data analyses were carried out with SPSS for Windows (version 16.0; SPSS Inc., Chicago, IL, USA), and a P-value < 0.05 was considered statistically significant.
4. Results
4.1. Patients’ Characteristics
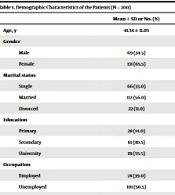
Table 1 outlines the demographic characteristics of the patients. The average age and disease duration of the patients was 41.43 ± 11.03 and 6.98 ± 5.76 years, respectively. Of the patients, 65.5% were female, 56.0% were married, 55.5% were university-educated, 39.0% were employed, 85.0% were residents in an urban area, 31.0% had a chronic disease, 90.5% had insurance, and 70.0% had RRMS.
| Mean ± SD or No. (%) | |
|---|---|
| Age, y | 41.34 ± 11.03 |
| Gender | |
| Male | 69 (34.5) |
| Female | 131 (65.5) |
| Marital status | |
| Single | 66 (33.0) |
| Married | 112 (56.0) |
| Divorced | 22 (11.0) |
| Education | |
| Primary | 28 (14.0) |
| Secondary | 61 (30.5) |
| University | 111 (55.5) |
| Occupation | |
| Employed | 78 (39.0) |
| Unemployed | 101 (50.5) |
| Retired | 21 (10.0) |
| Place of residence | |
| Urban | 170 (85.0) |
| Rural | 30 (15.0) |
| Chronic disease | |
| No | 138 (69.0) |
| Yes | 63 (31.0) |
| Insurance | |
| No | 19 (9.5) |
| Yes | 181 (90.5) |
| Supplementary insurance | |
| No | 103 (51.5) |
| Yes | 97 (48.5) |
| Disease duration | 6.98 ± 5.76 |
| Type of MS | |
| CIS | 6 (3.0) |
| RRMS | 140 (70.0) |
| PPMS | 10 (5.0) |
| SPMS | 29 (14.5) |
| PRMS | 15 (7.5) |
Demographic Characteristics of the Patients (N = 200)
4.2. Descriptive Statistics and Correlations Among Study Variables
Table 2 presents the means, SDs, and correlations among the study variables. The mean physical impact and psychological impact scores were 20.2 ± 22.9 and 31.6 ± 26.3, respectively. The MSIS-29 subscales were positively correlated with the PSS-4, GAD-7, and PHQ-9 scores (r ranging from 0.538 to 0.867, all P < 0.001).
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| 1 Stress (PSS-4) | 1 | ||||
| 2 Anxiety (GAD-7) | 0.693 | 1 | |||
| 3 Depression (PHQ-9) | 0.649 | 0.881 | 1 | ||
| 4 Physical impact (MSIS-29) | 0.538 | 0.711 | 0.743 | 1 | |
| 5 Psychological impact (MSIS-29) | 0.749 | 0.867 | 0.862 | 0.763 | 1 |
| Possible range | 0 - 16 | 0 - 21 | 0 - 27 | 0 - 100 | 0 - 100 |
| Observed range | 0 - 16 | 0 - 21 | 0 - 27 | 0 - 95 | 0 - 100 |
| Mean ± SD | 5.67 ± 3.97 | 6.33 ± 5.34 | 7.30 ± 6.14 | 20.2 ± 22.9 | 31.6 ± 26.3 |
Means, Standard Deviations, and Correlations Among Study Variables (N = 200) a
4.3. Relationship of MSIS-29 Scores with Demographic Variables
Table 3 shows the relationships between the physical impact and psychological impact scores and demographic variables among patients with MS using univariable analysis. There were positive correlations between age and scores of physical impact (r = 0.346, P < 0.001) and psychological impact (r = 0.220, P = 0.002). Similar but stronger correlations were observed between disease duration and scores of physical impact (r = 0.506, P < 0.001) and psychological impact (r = 0.368, P = 0.002). Single patients exhibited lower scores of physical and psychological impact than married or divorced patients. Overall, patients who had PRMS and SPMS subtypes obtained higher scores of physical and psychological impact than patients with CIS and RRMS. Retired subjects or patients with absenteeism reported higher physical and psychological impact scores.
| Physical Impact | Psychological Impact | |||
|---|---|---|---|---|
| Mean ± SD or r | P-Value | Mean ± SD or r | P-Value | |
| Age | 0.346 | < 0.001 | 0.220 | 0.002 |
| Gender | 0.996 | 0.860 | ||
| Male | 20.2 ± 22.1 | 31.2 ± 26.6 | ||
| Female | 20.2 ± 23.4 | 31.8 ± 26.2 | ||
| Marital status | 0.032 | 0.019 | ||
| Single | 14.3 ± 21.0 | 24.5 ± 23.7 | ||
| Married | 23.6 ± 24.1 | 34.4 ± 27.6 | ||
| Divorced | 20.6 ±18.8 | 39.0 ± 22.5 | ||
| Education | 0.148 | 0.003 | ||
| Primary | 25.7 ± 26.9 | 35.6 ± 29.7 | ||
| Secondary | 22.6 ± 22.2 | 39.7 ± 25.9 | ||
| University | 17.5 ± 22.0 | 26.2 ± 24.4 | ||
| Occupation | < 0.001 | 0.011 | ||
| Employed | 18.1 ± 20.8 | 29.1 ± 24.4 | ||
| Unemployed | 16.9 ± 20.9 | 30.2 ± 25.9 | ||
| Retired | 44.1 ± 26.7 | 47.8 ± 30.0 | ||
| Place of residence | 0.081 | 0.123 | ||
| Urban | 19.0 ± 23.5 | 30.4 ± 26.7 | ||
| Rural | 27.0 ± 18.1 | 38.4 ± 22.5 | ||
| Chronic disease | 0.099 | 0.173 | ||
| No | 18.4 ± 22.5 | 29.9 ± 26.5 | ||
| Yes | 24.2 ± 23.4 | 35.4 ± 25.5 | ||
| Insurance | 0.126 | 0.055 | ||
| No | 12.6 ± 20.3 | 20.6 ± 28.7 | ||
| Yes | 21.0 ± 23.1 | 32.8 ± 25.8 | ||
| Supplementary insurance | 0.173 | 0.825 | ||
| No | 18.1 ± 21.5 | 32.0 ± 26.9 | ||
| Yes | 22.5 ± 24.2 | 31.2 ± 25.7 | ||
| Disease duration | 0.506 | < 0.001 | 0.368 | < 0.001 |
| Type of MS | < 0.001 | < 0.001 | ||
| CIS | 22.5 ± 38.5 | 32.4 ± 38.4 | ||
| RRMS | 12.5 ± 16.2 | 25.7 ± 22.3 | ||
| PPMS | 29.4 ± 25.3 | 33.1 ± 18.1 | ||
| SPMS | 41.1 ± 21.1 | 46.2 ± 27.1 | ||
| RPMS | 44.7 ± 29.1 | 57.8 ± 33.4 | ||
Relationship of Multiple Sclerosis Impact Scale (MSIS-29) Scores with Demographic Variables Among Patients with Multiple Sclerosis (MS)
4.4. Multivariable Analysis
Hierarchical multiple linear regression analyses were performed to examine the variables associated with the MSIS-29 scores (Table 4). Regarding physical impact, in block 1, among demographic variables, disease duration was positively correlated with physical impact scores (b = 1.10, P < 0.001). Patients with PRMS, SPMS, and PPMS subtypes reported higher physical impact scores than those with RRMS. When the demographic and clinical variables were included in the model, the model R2 was 0.454, indicating that 45.4% of the variance in the physical impact scores was explained by these variables. In block 2, GAD-7 (b = 1.00, P = 0.012) and PHQ-9 (b = 1.43, P < 0.001) scores were positively correlated with physical impact scores. When the PSS-4, GAD-7, and PHQ-9 scores were added to the model, there was a considerable improvement in the model (R2 = 72.8%, ∆R2 = 27.4%, F(20,199) = 23.93, P < 0.001).
| Physical Impact | Psychological Impact | |||
|---|---|---|---|---|
| b (SE) | P-Value | b (SE) | P-Value | |
| Block 1 | ||||
| Age | -0.05 (0.20) | 0.789 | -0.43 (0.26) | 0.097 |
| Gender | ||||
| Male | Ref | Ref | ||
| Female | 3.17 (3.03) | 0.298 | 2.71 (3.96) | 0.494 |
| Marital status | ||||
| Single | Ref | Ref | ||
| Married | -0.92 (3.38) | 0.787 | 2.65 (4.41) | 0.549 |
| Divorced | 4.76 (4.86) | 0.328 | 12.62 (6.34) | 0.048 |
| Education | ||||
| Primary | Ref | Ref | ||
| Secondary | -8.88 (4.30) | 0.041 | -3.15 (5.62) | 0.576 |
| University | -8.43 (4.55) | 0.066 | -13.29 (5.94) | 0.027 |
| Occupation | ||||
| Employed | Ref | Ref | ||
| Unemployed | -6.08 (3.22) | 0.061 | -7.17 (4.21) | 0.090 |
| Retired | 9.97 (5.10) | 0.052 | 3.16 (6.66) | 0.636 |
| Place of residence | ||||
| Urban | Ref | Ref | ||
| Rural | 6.53 (3.74) | 0.083 | 7.00 (4.89) | 0.154 |
| Chronic disease | ||||
| No | Ref | Ref | ||
| Yes | -6.72 (3.34) | 0.046 | -4.28 (4.37) | 0.329 |
| Insurance | ||||
| No | Ref | Ref | ||
| Yes | 5.89 (4.59) | 0.200 | 13.11 (5.99) | 0.030 |
| Supplementary insurance | ||||
| No | Ref | Ref | ||
| Yes | 3.19 (2.85) | 0.264 | -1.89 (3.72) | 0.613 |
| Disease duration | 1.10 (0.30) | < 0.001 | 1.15 (0.39) | 0.004 |
| Type of MS | ||||
| CIS | Ref | Ref | ||
| RRMS | 0.67 (7.75) | 0.932 | 2.71 (10.12) | 0.789 |
| PPMS | 13.72 (6.12) | 0.026 | 2.09 (7.99) | 0.794 |
| SPMS | 20.36 (4.31) | < 0.001 | 15.52 (5.62) | 0.006 |
| RPMS | 30.05 (5.28) | < 0.001 | 29.18 (6.90) | < 0.001 |
| Model characteristics | R2 = 45.4%, F = 8.90, P < 0.001 | R2 = 29.2%, F = 4.41, P < 0.001 | ||
| Block 2 | ||||
| Stress | -0.09 (0.35) | 0.791 | 1.74 (0.30) | < 0.001 |
| Anxiety | 1.00 (0.40) | 0.012 | 1.56 (0.34) | < 0.001 |
| Depression | 1.43 (0.31) | < 0.001 | 1.52 (0.27) | < 0.001 |
| Model characteristics | R2 = 72.8%, ∆R2 = 27.4%, F = 23.93, P < 0.001 | R2 = 84.7%, ∆R2 = 55.5%, F = 49.43, P < 0.001 | ||
Factors Associated with Multiple Sclerosis Impact Scale (MSIS-29) Scores Among Patients with Multiple Sclerosis (MS) a
Regarding psychological impact, in block 1, among demographic variables, disease duration was positively correlated with psychological impact scores (b = 1.15, P = 0.004). Patients with PRMS and SPMS subtypes reported higher psychological impact scores than those with RRMS. Divorced patients exhibited higher scores of psychological impact than single patients. Patients with university education had significantly lower psychological impact scores than patients with primary education. Patients who had insurance reported higher psychological impact scores. The R2 in this block was 0.292, indicating that 29.2% of the variance in the psychological impact score was explained by the demographic and clinical variables. In block 2, all PSS-4 (b = 1.74, P < 0.001), GAD-7 (b = 1.56, P < 0.001), and PHQ-9 (b = 1.52, P < 0.001) scores were positively correlated with psychological impact scores. When the PSS-4, GAD-7, and PHQ-9 scores were added to the model, there was a considerable improvement in the model (R2 = 84.7%, ∆R2 = 55.5%, F(20,199) = 49.43, P < 0.001). More specifically, an additional 55.5% of the variance in the psychological impact score was explained by the PSS-4, GAD-7, and PHQ-9 scores.
5. Discussion
This study attempted to assess the quality of life in MS patients using the MSIS-29 quality of life measuring instrument to ascertain the quality of life and its predictors in individuals with MS. Additionally, the type of MS and its connection to other factors were taken into account in this study. The results of the study indicated that the score of physical and psychological impact in rural areas was higher than in urban areas, which can be due to various reasons, such as limited access to healthcare services, low socioeconomic level, and lack of sufficient education in these areas, which have been mentioned in previous studies (41-43).
According to the data, the psychological impact score was greater than the physical impact score. The findings of a study by McKay et al. in this respect also demonstrated that the psychological impact score in the study population is greater than the physical impact score (44). However, according to a study by Dymecka and Bidzan participants reported the psychological aspect of their quality of life better (45). The results of the current study indicated that stress, rather than other psychological factors, such as anxiety and depression, has a bigger effect on the quality of life of these patients. However, the impact of depression and anxiety was equally notable and substantial. This is even though that stress was not a reliable predictor in another study in 2006 (15). Additionally, according to the results, the effect of these three variables on the psychological dimension was more than the physical dimension.
Patients with progressive MS in the current study reported a decreased quality of life and higher anxiety and depression. According to a study’s findings, physical disability has a more negative impact on the quality of life (46). Therefore, it seems that the cause of this finding in this group of patients is more experience with a physical disability.
According to the results, the relationship between the duration of the disease and the score of physical and psychological impact was stronger than age. Primary progressive MS, SPMS, and PRMS patients were shown to have higher physical and psychological impact scores than RRMS patients. It seems that one of the reasons for this finding is the existence of recovery periods in the RRMS type and the lower average age in these individuals. As shown in a study by Rooney et al., individuals with progressive MS were older, and more time had passed since the diagnosis of the disease (47). However, type RRMS individuals, based on research in 2020, reported greater levels of anxiety and depression (27). Demonstrating whether type RRMS patients have better circumstances than other groups relies on many aspects and needs further studies in a larger population.
According to the findings of a study by Rosiak and Zagozdzon the RRMS stage begins sooner in women than in men. Additionally, men are diagnosed with the progressive form of MS earlier (48); then, social support is crucial for both groups. Accordingly, some studies show that men received less support (49); however, the significant impact of social support on the quality of life of MS patients has been proven (50, 51).
In addition, in a study by Schwartz and Frohner it was shown that cognitive impairment will be less in patients with longer disease duration and patients with greater perceived social support. It was also observed that these patients had better mental health (52).
The present study showed that singles had a greater quality of life and lower levels of anxiety and depression than married and divorced patients. This might be because single individuals are often younger than married individuals and have fewer obligations. A different study demonstrated that single individuals had less anxiety and depression (27).
Individuals with university educations exhibited lower levels of anxiety and depression in the current study, comparable to Yalachkov’s study (53); it seems they are more conscious, have made better lifestyle choices, and have better self-care (54-56). It makes the value of information and education more obvious.
In this study, it was observed that retired individuals obtained a much higher score than other groups, both physically and psychologically, and they have a lower quality of life, as in a study by Marck et al. (57). This might be due to less social communication and distance from the work environment and the higher average age of these individuals since retired individuals have a higher average age than other groups. On the other hand, increasing age is associated with an increase in the probability of hospitalization (58). Therefore, because with increasing age, the probability of contracting other chronic diseases increases and the chances of being admitted to the hospital multiply, monitoring the health status of patients in terms of other chronic diseases becomes more important, and this action can have a positive effect on the quality of life of these patients.
The researchers suggest a more focused analysis in a larger population since the psychological effect score in the present study was greater in female subjects than in male cases, which is likely related to the study population’s limitations.
5.1. Conclusions
In general, based on the results of the present study, it can be said that MS is one of the diseases that can affect the quality of life of a person in both physical and mental aspects. In the meantime, various factors, including individual variables (e.g., patient age and marital status), psychological factors (e.g., stress, anxiety, and depression), and disease-related factors (e.g., the duration of the disease), can affect the quality of life of these patients. It seems that these patients need special support and attention in all dimensions because all these factors can affect the consequences of the disease. As a result, these patients must continue to obtain the required follow-ups and training to enhance self-care; accordingly, they can effectively adjust to the disease’s signs and symptoms. To see an improvement in this group of patient’s quality of life, it is proposed that healthcare systems should set up programs to help these patients as much as possible in both physical and mental aspects.
Therefore, it seems that considering the greater communication of nurses with these patients in inpatient and outpatient clinical environments and in associations and institutions that support patients, nurses need to pay special attention to the variables that affect the quality of life of these patients and take steps to adjust the factors related to reducing the quality of life. Among the nursing measures in this direction are regular follow-up of these patients, provision of psychological counseling by specialized nurses or psychologists, and proper referral of patients based on their clinical conditions. On the other hand, considering the role of demographic and clinical variables in the quality of life, it seems that nurses should provide unique care for each of these patients to improve their quality of life.
5.2. Nursing Implications
This study has some implications for healthcare providers, especially nurses and MS patients. The results of the present study, which were used to determine the predictors of quality of life in patients with MS, can be used in the planning of healthcare providers, including nurses. Considering that the results show that psychological variables, such as anxiety, depression, and perceived stress, have an effect on the physical and mental dimensions of the quality of life of these patients, it seems necessary that nurses should include consultations in the care planning of these patients. This can help improve the quality of life of these patients.
5.3. Limitations
This study has a few limitations in generalizing the results because the sample was selected from a single treatment center. Future studies can be performed in various places, such as other provinces of Iran. Additionally, another limitation of this project was the low willingness of MS patients to participate in the research project due to fatigue caused by problems related to their disease; for the reduction of this problem, data collection was conducted in several stages.