1. Background
COVID-19, a respiratory tract infection caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become a global pandemic (1). This infection has led to high rates of adverse outcomes and mortality, with severe and critical disease occurring in 14% and 5% of cases, respectively (2). Individuals with chronic conditions, such as type 2 diabetes mellitus (T2DM), face a higher risk of mortality from COVID-19 (3, 4), with studies indicating a mortality rate of 20 - 30% among those with diabetes who contract the virus (5-7). Furthermore, hyperglycemia has been identified as a strong predictor of a poor prognosis in patients with COVID-19 (8).
Severe complications of COVID-19 commonly include acute respiratory distress syndrome (ARDS), heart failure, respiratory failure, acute cardiac injury, and sepsis (9). Diabetic patients are more likely to experience severe symptoms and complications from COVID-19; however, with well-controlled diabetes, the risk of acute symptoms is comparable to that of individuals without diabetes (10).
The primary treatment for T2DM involves antidiabetic medications such as metformin and gliclazide, which improve insulin sensitivity, suppress gluconeogenesis, and enhance glucose absorption in tissues (11).
Dipeptidyl peptidase-4 (DPP-4) inhibitors, representing a newer class of antihyperglycemic drugs, have been recognized for their efficacy and safety. Sitagliptin, a key DPP-4 inhibitor, has been shown to inhibit the inflammatory response of the immune system and decrease serum levels of inflammatory factors in diabetics (12-15).
The angiotensin-converting enzyme-2 (ACE2) is utilized by SARS-CoV-2 as a receptor to enter host cells. Sitagliptin has been observed to block the virus's entry by activating ACE2 through AMPK signaling, thereby reducing adverse outcomes (16-18). Additionally, previous studies have highlighted the role of metformin in decreasing COVID-19-related mortality (19, 20).
In Iran, where diabetes prevalence is relatively high, there is an increased rate of mortality and adverse outcomes from COVID-19 among diabetic individuals (21, 22). Sitagliptin and metformin are widely used medications for managing T2DM, yet their specific effects on COVID-19 outcomes in the Iranian population have not been extensively studied.
2. Objectives
Thus, this research was undertaken to assess the frequency of mortality and adverse outcomes in T2DM patients previously treated with sitagliptin, metformin, or both.
3. Methods
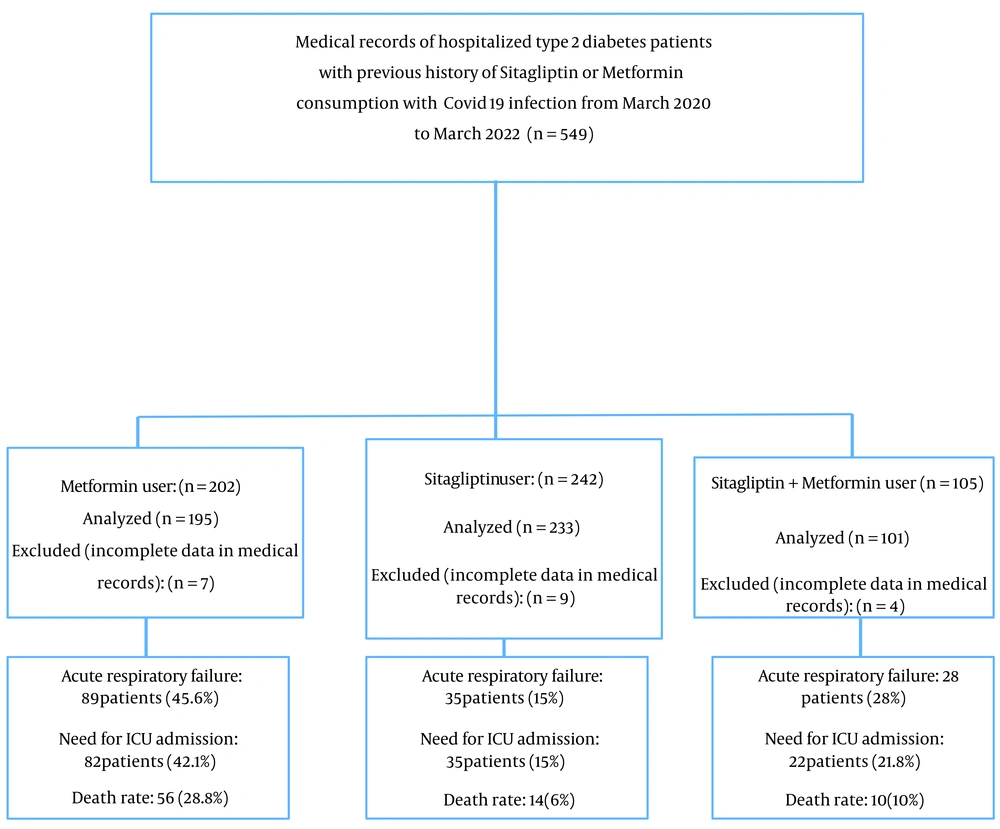
This retrospective study reviewed the medical records of T2DM patients diagnosed with COVID-19 who had previously received treatment with sitagliptin, metformin, or both and were admitted to Naft Hospital in Ahvaz, Iran, from March 2020 to March 2022. We included all medical records of diabetic patients diagnosed with COVID-19 who had a history of using sitagliptin, metformin, or both for at least three months (N = 549). Medical records lacking complete information were excluded (n = 20). Informed consent was obtained from all patients at the beginning of hospitalization. The Ethics Committee of Ahvaz Jundishapur University of Medical Sciences in Ahvaz, Iran, approved this study (Ref. ID: IR.AJUMS.REC.1400.534). The flowchart of the study I displayed in Figure 1.
Confirmation of COVID-19 infection was based on the reverse transcription-polymerase chain reaction (RT-PCR) tests of throat and nasal swab specimens and/or characteristic chest computed tomography (CT) scan findings, such as distributed multifocal ground-glass opacities (GGO) and patchy consolidation in suspected patients. Data extracted from the medical records included demographic and primary data (age, sex, duration of diabetes, underlying diseases), length of hospital stay, mortality, and adverse outcomes during hospitalization, including acute respiratory failure, myocardial infarction, hemorrhagic stroke, pulmonary embolism, and the need for ICU admission.
Acute respiratory failure was confirmed through venous blood gas (VBG) tests, arterial blood oxygen saturation levels, the necessity for intubation, and myocardial infarction, determined by electrocardiogram (ECG) results, positive troponin levels, and cardiac consultations. Pulmonary embolism diagnoses were confirmed by CT lung angiography and lung consultations, while hemorrhagic stroke diagnoses were based on imaging evidence and neurology consultations. Additionally, hypertension was identified in patients with blood pressure equal to or exceeding 130/80 mmHg or those on antihypertensive medication. Dyslipidemia was determined based on serum triglyceride levels > 200 mg/dL, total cholesterol > 200 mg/dL, low-density lipoprotein (LDL)-cholesterol > 100 mg/dL, high-density lipoprotein (HDL)-cholesterol < 45 mg/dL in men and < 54 mg/dL in women, or the use of lipid-lowering drugs.
Quantitative variables were summarized using mean and standard deviation (SD). The Kolmogorov-Smirnov test assessed the normal distribution of quantitative variables. ANOVA was utilized to compare mean variables, while logistic regression models identified risk factors associated with mortality and ICU admission. Qualitative variables were described using frequency and percentage. The chi-square test was applied to compare qualitative variables. P-values less than 0.05 were deemed statistically significant. Statistical analyses were conducted using SPSS (SPSS Inc., Chicago, IL, USA) version 22.
4. Results
In this study, we analyzed 529 medical records of hospitalized patients. The age range of the patients was from 24 to 95 years, with a mean and standard deviation of their age being 64.06 ± 9.4. The cohort comprised 284 men (53.7%) and 245 women (46.3%). The average duration of type 2 diabetes among the patients was 11.85 ± 5.39 years, with a median of 10 years (range 1 - 38 years). The average length of hospital stay was 5.88 ± 4.27 days, with a median of 5 days (range 1 - 30 days). Data extracted from the medical records revealed that the median glycated hemoglobin (HbA1C) level was 9.2% (mean ± SD: 8.9 ± 0.6%).
A total of 195 (36.87%) patients were on metformin, 233 (44.04%) on sitagliptin, and 101 (19.09%) were taking both metformin and sitagliptin. Table 1 shows the basic characteristics of patients across the three treatment groups. Compared to the other two groups, patients only on Metformin had a significantly higher frequency of underlying diseases such as hypertension and dyslipidemia, and a longer history of taking other oral antidiabetic drugs (e.g., gliclazide, repaglinide, and pioglitazone) (P < 0.0001).
| Variables | Metformin (n = 195) | Sitagliptin (n = 233) | Sitagliptin + Metformin (n = 101) | P-Value |
|---|---|---|---|---|
| Age ≥ 65 years | 109 (55.9) | 121 (51.9) | 47 (46.5) | 0.306 |
| Gender, male | 103 (52.8) | 129 (55.4) | 52 (51.5) | 0.771 |
| Duration of diabetes ≥ 10 years | 132 (67.7) | 154 (66.1) | 64 (63.4) | 0.757 |
| History of hypertension | 172 (88.2) | 111 (47.8) | 70 (69.3) | < 0.0001 |
| History of dyslipidemia | 124 (63.9) | 70 (30.2) | 58 (58) | < 0.0001 |
| Other oral antidiabetic drugs | 80 (41) | 63 (27) | 65 (64.4) | < 0.0001 |
| Insulin | 16 (8.2) | 27 (11.6) | 5 (5) | 0.132 |
| Length of hospital stay, day | 6.59 ± 5.17 | 5.47 ± 3.39 | 5.44 ± 4.01 | 0.014 |
a Values are expressed as No. (%) or mean ± SD.
The overall mortality rate among diabetic patients was 15.1% (80 patients). Additionally, respiratory failure was observed in 152 (28.8%) patients, myocardial infarction in 31 (5.9%), pulmonary embolism in 141 (26.7%), the need for ICU admission in 136 (25.7%), and stroke in 29 patients (5.5%). The frequency of COVID-19-induced mortality and adverse outcomes among diabetics in the three treatment groups is presented in Table 2.
| Variables | Metformin (n = 195) | Sitagliptin (n = 233) | Sitagliptin + Metformin (n = 101) | P-Value |
|---|---|---|---|---|
| Death | 56 (28.8) | 14 (6) | 10 (10) | < 0.0001 |
| Acute respiratory failure | 89 (45.6) | 35 (15) | 28 (28) | < 0.0001 |
| Myocardial infarction | 15 (7.7) | 11 (4.7) | 5 (5) | 0.390 |
| Stroke | 19 (9.7) | 4 (1.7) | 6 (6) | 0.01 |
| Pulmonary embolism | 76 (39) | 34 (14.6) | 31 (30.7) | < 0.0001 |
| Need for ICU admission | 82 (42.1) | 35 (15) | 22 (21.8) | < 0.0001 |
a Values are expressed as No. (%).
Logistic regression models were utilized to determine risk factors affecting mortality, the need for ICU admission, acute respiratory failure, and pulmonary embolism among the studied patients.
The use of Sitagliptin, alone or in combination with Metformin, significantly reduced the mortality rate in diabetic individuals with COVID-19 (P < 0.001), with Sitagliptin alone showing a more significant reduction in death rate (P < 0.0001). Additionally, the duration of diabetes significantly increased the mortality rate.
A history of hypertension (OR = 3.14, 95% CI: 1.48 - 6.66, P = 0.003) and previous Metformin use (OR = 3.57, 95% CI: 2.02 - 7.57, P < 0.0001) were identified as significant risk factors for mortality. A history of hypertension was also a significant risk factor for ICU admission (OR = 3.183, 95% CI: 1.608 - 6.300, P = 0.001) as shown in Table 3.
| Variables | ICU Admission | Mortality | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI for OR | P-Value | OR | 95% CI for OR | P-Value | |
| Gender (male/female) | 0.94 | 0.62 - 1.43 | 0.79 | 0.848 | 0.50 - 1.41 | 0.52 |
| Age > 65 years | 1.35 | 0.86 - 2.10 | 0.18 | 1.692 | 0.972 - 2.94 | 0.063 |
| Duration of diabetes | 1.97 | 1.2 - 3.24 | 0.007 | 2.194 | 1.15 - 4.18 | 0.017 |
| Hypertension | 3.183 | 1.608 - 6.300 | 0.001 | 3.14 | 1.48 - 6.66 | 0.003 |
| Dyslipidemia | 1.321 | 0.777 - 2.246 | 0.303 | 1.084 | 0.493 - 2.385 | 0.840 |
| Drug (sitagliptin/metformin) | 0.239 | 0.15 - 0.38 | < 0.0001 | 0.155 | 0.083 - 0.291 | < 0.0001 |
| Drug (sitagliptin + metformin) | 0.393 | 0.22 - 0.68 | 0.001 | 0.280 | 0.13 - 0.58 | 0.001 |
Sitagliptin was found to reduce the risk of respiratory failure and pulmonary embolism compared to Metformin. The combination of metformin and sitagliptin also effectively reduced side effects compared to metformin alone, although its effectiveness was less compared to sitagliptin alone, as detailed in Table 4.
| Variables | Respiratory Failure | Pulmonary Emboli | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI for OR | P-Value | OR | 95% CI for OR | P-Value | |
| Gender (male/female) | 1.04 | 0.69 - 1.56 | 0.83 | 0.83 | 0.56 - 1.25 | 0.393 |
| Age > 65 years | 1.318 | 0.856 - 2.03 | 0.210 | 0.978 | 0.635 - 1.50 | 0.920 |
| Duration of diabetes | 1.758 | 1.09 - 2.82 | 0.019 | 1.204 | 0.76 - 1.90 | 0.426 |
| Drug (sitagliptin/metformin) | 0.207 | 0.130 - 0.329 | < 0.0001 | 0.268 | 0.169 - 0.427 | < 0.0001 |
| Drug (sitagliptin+ metformin) | 0.478 | 0.283 - 0.810 | 0.006 | 0.695 | 0.416 - 1.16 | 0.165 |
5. Discussion
Based on the findings of this study, the mortality rate among hospitalized T2DM patients with COVID-19 infection stands at 15.1%. Smati et al. reported a COVID-19-induced mortality rate of 20.6% in diabetic patients (5). Bhinder et al. documented a mortality rate of 47%, possibly due to a smaller sample size (23). De-la-Rosa-Martinez et al. noted a 34% mortality rate among diabetic patients infected with COVID-19 (6), while Gajecki et al. observed a COVID-19-induced mortality rate of 25% among diabetic patients (7). The discrepancy in mortality rates across these studies could stem from differences in sample selection and the period of the study.
In this study, the mortality and adverse outcomes were found to be lower among those who had previously used sitagliptin compared to those who had taken metformin. Specifically, the mortality rates were lower in the sitagliptin (6%) and the sitagliptin plus metformin (10%) groups than in the metformin alone group (28.8%). Gao et al. determined that life-threatening complications in hospitalized T2DM patients with COVID-19 were more common among metformin users than non-users (7.4% vs. 28.6%, P = 0.004) (24).
A 2021 meta-analysis by Li et al., which reviewed 19 studies, indicated a 34% reduction in mortality and a 27% decrease in hospitalization rates for COVID-19 patients who took metformin (25). Kan et al. found that the use of metformin and sulfonylurea was linked to a lower risk of mortality in T2DM patients with COVID-19 (26). Treatment with metformin in diabetic patients with COVID-19 was associated with fewer complications, such as inflammation, renal ischemia, thrombosis, and shorter hospital stays (20, 25). Samuel et al. highlighted the specific role of metformin in lowering COVID-19-associated mortality (19).
Nevertheless, this study's results indicate significant differences in COVID-19 complications—including acute respiratory failure, hemorrhagic stroke, pulmonary embolism, ICU admission, and intubation—across the three treatment groups. Patients on metformin experienced the highest frequency of complications and COVID-19-induced adverse outcomes, while those in the sitagliptin group had fewer complications. These outcomes suggest that sitagliptin, associated with lower mortality, might be effective in managing COVID-19 in diabetic patients.
Sitagliptin not only lowers blood glucose levels but also exhibits anti-inflammatory (14, 15) and immunomodulatory effects (26). Considering the elevated levels of inflammatory factors in COVID-19 (27, 28), sitagliptin could help mitigate complications and the disease's severity. It activates AMPK through liver kinase B1 (LKB1) and inhibits the mammalian target of rapamycin (mTOR) pathway (29), indirectly reducing AKT activation and the mTOR signaling cascade. Given the significant role of the AKT-mTOR phosphoinositide 3-kinase (PI3K) pathway in MERS-CoV infection, the potential of sitagliptin against SARS-CoV-2 has attracted scholarly interest (18, 30).
Bardaweel et al. proposed that sitagliptin, whether used alone or in combination with other medications, might aid in treating COVID-19 in diabetic patients with heart disease (18). However, this study did not focus specifically on diabetics with heart disease. Solerte et al.'s retrospective study demonstrated that T2DM patients with COVID-19 treated with sitagliptin had superior outcomes compared to those on standard therapy. The sitagliptin group experienced a lower mortality rate (18%) and a higher rate of clinical improvement (60%) than the standard treatment group (37% mortality rate; 38% clinical improvement) (31). Abbasi et al. highlighted the potential benefits of sitagliptin in enhancing clinical outcomes in hospitalized COVID-19 patients (32). Mirani et al. identified a significant and independent link between the use of DPP-4 inhibitors and a reduced mortality risk (33). These studies support the findings of the current study, suggesting that sitagliptin may yield better outcomes than standard treatments.
However, Fadini et al. found no evidence to suggest that DPP-4 inhibitors are linked to hospitalization due to COVID-19 (34). Nonetheless, the present study discovered that sitagliptin effectively shortened hospital stays. Variations among studies might be attributed to differences in study populations, the characteristics of the participants, the presence of underlying diseases, and sample sizes.
While prior research has established the link between diabetes and an increased incidence and severity of COVID-19, managing glucose and treating diabetes have been shown to decrease COVID-19-induced mortality in diabetics (35). This study indicates that sitagliptin, as opposed to metformin, is associated with a reduced frequency of death and COVID-19 complications. Sitagliptin's complex mechanism of action, including its anti-inflammatory properties, may contribute to this reduced risk of severe COVID-19.
This study faces several limitations, including reliance on a retrospective analysis of medical records, which could introduce biases and data collection limitations. It also lacked a control group of diabetic patients not using sitagliptin or metformin, complicating direct outcome comparisons. Additionally, the absence of follow-up for survivors might have offered more insight into long-term effects and outcomes. To address these limitations and obtain more precise results, future prospective studies with a control group and sufficient follow-up are recommended. This approach will provide more reliable and comprehensive insights into the benefits of sitagliptin for T2DM patients with COVID-19.
5.1. Conclusions
Our study concludes that hospitalized T2DM patients with COVID-19 infection who were using sitagliptin had a lower mortality rate compared to those using metformin alone. Furthermore, a higher rate of adverse outcomes, including acute respiratory failure, stroke, pulmonary embolism, ICU admission, and intubation, was observed in the metformin alone group compared to the other two groups. These findings imply that sitagliptin usage in patients with type 2 diabetes and COVID-19 may help mitigate the risk of death and adverse outcomes.