1. Background
In recent years, the prevalence of kidney stone disease (KSD) has been increasing, ranging between 4% and 20% in developing countries (1). It is estimated that the recurrence rate of urinary stones will reach approximately 50% within the next 10 years. The likelihood of kidney stone formation during one's lifetime is 10% for men and 6% for women, with an increasing trend, particularly in many developed countries (2).
Various multifactorial risk factors contribute to kidney stones, including age, sex, race/ethnicity, climate, and occupation (3). Additionally, nephrolithiasis is associated with other conditions such as arterial hypertension, diabetes mellitus, and metabolic syndrome (2), with obesity being a common risk factor for several diseases, including KSD (4). The composition of one's diet also plays a crucial role in urine risk profile (5). Consequently, stone formation in obese individuals is linked to factors such as insulin resistance, poor dietary habits, and other metabolic factors that contribute to a higher lithogenic urinary profile (3).
The most prevalent types of kidney stones in humans include calcium oxalate and calcium phosphate, or a combination of both (6). Compared to individuals of normal weight, those who are obese excrete higher levels of calcium, citrate, uric acid, sodium, potassium, magnesium, and creatinine, as well as experience higher calcium phosphate and uric acid saturation levels in their urine (3). Ultimately, factors such as inadequate fluid intake, high temperatures, or excessive consumption of foods rich in oxalates contribute to the formation of KSD (7). Uric acid precipitation in urine and the formation of KSD result from defects in renal ammonia genesis and acidic urine pH, often associated with insulin resistance (8).
Previous studies have demonstrated an association between dyslipidemia and KSD, with a higher prevalence of dyslipidemia observed in patients with stone formation compared to those without stones (9). Similarly, a study conducted in Turkey found that individuals with KSD had significantly higher levels of total cholesterol, and most KSD cases consisted of calcium oxalate mono-dihydrate and uric acid stones. This study suggests that factors such as BMI, hypercholesterolemia, and hyperlipidemia play pivotal roles in metabolic syndrome and may be linked to KSD (10).
Furthermore, a study conducted in Taiwan investigated the relationship between KSD and obesity-related indicators from December 2008 to February 2020. Logistic regression analysis revealed that all obesity-related indicators, including BMI, waist circumference, waist-to-hip ratio, waist-to-height ratio, abdominal volume index, and body roundness index, were significantly associated with KSD. These indicators are considered prognostic factors for KSD (7). Another study in Taiwan suggested that metabolic syndrome is an increasing risk factor for KSD (11).
2. Objectives
Given that KSD is a multifactorial condition with varying prevalence rates among different populations, and considering the relatively high prevalence of this disease in Fasa, this study aimed to investigate the relationship between BMI, lipid profile, and kidney stones within the Fasa cohort.
3. Methods
3.1. Study Design and Participants
A cross-sectional study was conducted during the initial phase of the Fasa Adult Cohort Study (FACS). Fasa Adult Cohort Study is part of the Prospective Epidemiological Research Studies in Iran (PERSIAN), which are nationwide studies aimed at investigating prevalent noncommunicable diseases such as cardiovascular diseases, cancer, diabetes, metabolic syndrome, non-alcoholic fatty liver diseases, kidney stones, and others in Iran. The first phase of this cohort was carried out from 2014 to 2016, with subsequent annual follow-ups (12). This population-based study was conducted in the Shashada and Qara Balag regions, encompassing 41,000 individuals. Among this population, a total of 10,133 individuals aged between 35 and 70 years were included in the study through simple random sampling. Eligible participants were required to have resided in the area for at least 5 years. However, individuals who were unable to complete the measurements or questionnaire (due to mental or physical disorders) were excluded from the study (12).
3.2. Study Instruments and Variables Assessment
Each participant underwent an interview and completed a questionnaire approved by the PERSIAN cohort consortium in the Islamic Republic of Iran. The questionnaire collected sociodemographic information including sex, age, marital status, education level, employment history, socioeconomic status, and residential location through interviews and self-reports. Additionally, the clinical questionnaire gathered data on the participants' history of chronic diseases and medication use. Measurements of BMI, weight, and height were obtained for each participant. Weight was measured using a digital scale with an accuracy of 0.1 kg, while height was assessed using a stadiometer with an accuracy of 0.1 cm. BMI was calculated by dividing weight in kilograms by the square of height in meters (kg/m2). Blood samples were collected from each participant for lipid profile analysis, including low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides (TG), and cholesterol, using a colorimetric Pars Azmoon kit. The normal ranges for TG, cholesterol (CHOL), HDL, and LDL were less than 150 mg/dL, 200 mg/dL, 130 mg/dL, and above 40 mg/dL for men and 50 mg/dL for women, respectively. Additionally, systolic and diastolic blood pressure, as well as resting heart rate, were measured. Smoking, alcohol, and opium consumption histories were recorded, with participants asked about their usage of opium-derived products, alcohol, or if they were active smokers. Regression analysis was conducted to account for confounding factors such as age, gender, BMI, fat intake, medications, smoking, and alcohol consumption.
3.3. Statistical Analysis
Following written informed consent and obtaining permission and a research license from the department, data were collected from the FASA cohort for statistical analysis using SPSS 16. An independent-samples t-test was employed to examine the relationship between quantitative variables and the presence of kidney stones, while chi-square analysis was used to assess the association between qualitative variables and kidney stones. Logistic regression analysis was performed to identify confounding variables and calculate the odds ratio (P-value < 0.05).
4. Results
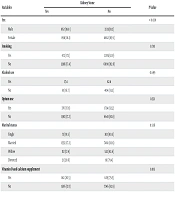
A total of 10,133 individuals participated in this study, comprising 4 575 (45.1%) men and 5,558 (54.9%) women. The mean age of the participants was 48.63 ± 9.57 years. The prevalence of kidney stones was 17.3%. Tables 1 and 2 present quantitative and categorical variables (as potential confounders) between two groups: Those with kidney stones and those without. Age, systolic blood pressure, diastolic blood pressure, dairy consumption, sex, and variables related to vitamin D and calcium supplements differed significantly between individuals with kidney stones and those without (P < 0.05).
| Variables | With Kidney Stone | Without Kidney Stone | P-Value |
|---|---|---|---|
| Age | 49.2 ± 9.644 | 48.47 ± 9.551 | ≤ 0.001 |
| Systolic blood pressure | 113.78 ± 19.071 | 110.9 ± 18.23 | ≤ 0.001 |
| Diastolic blood pressure | 75.87 ± 12.47 | 74.49 ± 11.79 | ≤ 0.001 |
| Education | 4.64 ± 3.963 | 4.66 ± 3.873 | 0.837 |
| Dairy consumption | 212.06 ± 179.55 | 203.75 ± 178.86 | 0.076 |
a Values are expressed as mean ± SD.
| Variables | Kidney Stone | P-Value | |
|---|---|---|---|
| Yes | No | ||
| Sex | < 0.001 | ||
| Male | 862 (18.8 ) | 37.13 (81.2) | |
| Female | 896 (16.1) | 4662 (83.9) | |
| Smoking | 0.710 | ||
| Yes | 472 (17.1) | 2285 (82.9) | |
| No | 1286 (17.4) | 6090 (82.6) | |
| Alcohol use | 0.699 | ||
| Yes | 17.4 | 82.6 | |
| No | 81 (16.7) | 404 (83.3) | |
| Opium use | 0.521 | ||
| Yes | 376 (17.8) | 1734 (82.2) | |
| No | 1382 (17.2) | 6641 (82.8) | |
| Marital status | 0.556 | ||
| Single | 73 (19.5) | 301 (80.5) | |
| Married | 1552 (17.2) | 7461 (82.8) | |
| Widow | 112 (17.4) | 532 (82.6) | |
| Divorced | 21 (20.6) | 81 (79.4) | |
| Vitamin D and calcium supplement | 0.015 | ||
| Yes | 162 (20.5) | 629 (79.5) | |
| No | 1596 (17.1) | 7746 (82.9) | |
a Values are expressed as No. (%).
Table 3 also compares the average Body Mass Index (BMI) and blood lipid levels between individuals with and without a history of kidney stones. It indicates that the average BMI and triglyceride levels are significantly higher in individuals with kidney stones compared to those without.
| Variables | With Kidney Stone | Without Kidney Stone | P-Value |
|---|---|---|---|
| Cholesterol, mg/dL | 186.22 ± 39.030 | 184.84 ± 39.204 | 0.178 |
| Triglyceride, mg/dL | 138.30 ± 79.936 | 130.48 ± 82.902 | < 0.001 |
| HDL, mg/dL | 50.98 ± 16.501 | 51.02 ± 15.773 | 0.914 |
| LDL, mg/dL | 107.58 ± 32.832 | 107.64 ± 32.925 | 0.952 |
| Body Mass Index, W(kg)/(H)2 | 26.272 ± 4.755 | 25.532 ± 4.828 | < 0.001 |
Table 4 displays the results of multivariable logistic regression analysis, showing that BMI and triglycerides are associated with kidney stones (adjusted for age, sex, dairy consumption, vitamin D and calcium supplementation, and systolic blood pressure). With each unit increase in BMI and triglyceride levels, the likelihood of having kidney stones increases by 1.039 (95% CI: 1.025 - 1.048) and 1.004 (95% CI: 1.001 - 1.006), respectively.
| Variables | OR | 95 CI for OR | P-Value a |
|---|---|---|---|
| Body Mass Index | 1.039 | 1.025 - 1.048 | < 0.001 |
| Triglyceride | 1.004 | 1.001 - 1.006 | 0.005 |
| Cholesterol | 1.001 | 0.999 - 1.011 | 0.316 |
| HDL | 1.001 | 0.997 - 1.003 | 0.976 |
| LDL | 1 | 0.998 - 1.001 | 0.875 |
a Adjusted for age, sex, diary consumption, vitamin D and calcium supplement and systole blood pressure.
5. Discussion
This study examined the relationship between lipid profiles and BMI with kidney stones. The findings revealed significant associations between triglyceride levels, BMI, and kidney stones. Obesity has long been recognized as a risk factor in numerous clinical trials. Taylor et al. conducted an epidemiological cohort study, reporting associations between weight, BMI over 30 kg/m², increased visceral adipose tissue, and the risk of developing calcium oxalate and uric acid kidney stones (9). Similar findings were observed in studies conducted in other countries, confirming that metabolic syndrome independently contributes to kidney stone formation. For instance, a 2019 - 2020 study in China found that 14.5% of patients with metabolic syndrome had kidney stones, compared to 7.95% of non-metabolic syndrome patients (10). Experimental studies in rats fed a high-fat diet, simulating obesity-related metabolic syndrome, demonstrated decreased urine pH and elevated levels of hyperoxalemia, hypercalciuria, triglycerides, blood sugar, and total cholesterol, all contributing to kidney stone formation and crystal deposition (13). Additionally, Yoshimura et al. identified BMI as an independent factor in the prevalence of kidney stones (14). Previous research has suggested that BMI influences risk factors such as reduced urine output and decreased citrate excretion in urine, contributing to kidney stone formation (15).
In alignment with our study findings, a 2021 study conducted in the Taiwanese population cohort elucidated the link between lipid profiles and kidney stones. Elevated triglyceride levels increased the risk of kidney stones by 1.46 times. Additionally, low HDL-C levels and a high cholesterol to HDL-C ratio further amplified this risk (16). A cohort follow-up study in the UK spanning from 1990 to 2007 revealed an increased risk of metabolic syndrome in kidney stone formation, particularly in southern England. Comparing individuals with metabolic syndrome to those without, the risk was nearly double in the former group. Another study in a UK cohort by Robert M. Geraghty in 2021 corroborated this finding, indicating that metabolic syndrome doubles the risk of developing KSD (17). Furthermore, two other studies indicated that dyslipidemia may trigger kidney stone formation and contribute to KSD (18, 19). The precise relationship between triglycerides, BMI, and kidney stone formation remains uncertain. One potential mechanism involves excess free fatty acids and lipotoxicity, which increase citrate excretion in proximal tubule cells, thereby interfering with glutamine consumption, reducing ammonia genesis for the blood buffer system, and affecting blood pH regulation. This leads to lower urine pH and crystal deposition in the kidney and urinary tract (20, 21). Consistent with other cohort studies and our own findings, KSD was found to be associated with triglyceridemia, high BMI, and low HDL, which are indicative of metabolic syndrome along with hypertension, obesity, and insulin resistance. According to the results, there was an increased expression of pro-inflammatory cytokines such as TNFα, IL-1β, and IL-6 in individuals exhibiting these characteristics, which stimulate kidney stone formation processes (19, 22, 23).
5.1. Strengths of the Study
This study utilized a population-based approach with a large sample size. Data collection was carried out by trained and experienced experts, and biochemical factors were assessed through laboratory tests, thereby enhancing the robustness of our findings.
5.2. Limitations
The study did not differentiate between various types of kidney stones, which may have different underlying causes. Further investigations into the specific types of kidney stones and their associations with different obesity-related factors, such as genetic predisposition, dietary patterns, and hypothyroidism, are warranted.
5.3. Conclusions
Based on our findings, KSD was correlated with elevated levels of triglycerides and BMI in the Fasa PERSIAN cohort. A high-fat diet is linked to increased BMI and elevated serum triglyceride levels, both of which are recognized risk factors for kidney stone formation.