1. Background
Coronavirus disease 2019 (COVID-19) infection was detected in late 2019 in Wuhan (China), and to date, 203 944 144 confirmed cases and 4 312 902 deaths from this disease have been recorded. Meanwhile, about five million cases of COVID-19 have been diagnosed in Iran, and more than 100 000 individuals have died up to now in this country (1).
The effect of COVID-19 infection on the level of endogenous hormones in the body has recently attracted much scholarly attention. One group of these hormones is thyroid hormones. Despite the importance of this topic, few studies have evaluated the changes in thyroid hormones in individuals affected with COVID-19. For instance, Chen et al. studied 50 individuals affected with COVID-19 and showed that the levels of thyroid-stimulating hormone (TSH) and triiodothyronine (T3) were significantly lower than those in healthy individuals (2). Confirming Wang et al.’s findings, Wang et al. also reported that even TSH level was much lower in patients with severe COVID-19 infection than in other patients (3). Lania et al. demonstrated that the function of the thyroid in most cases who were affected by COVID-19 infection remained normal; some cases showed thyrotoxicosis, and only a minority of the patients showed hypothyroidism (4).
A systematic review, including seven studies and 1 237 patients, showed that the prevalence of thyroid dysfunction varied from 13% to 64% in patients affected with COVID-19 infection (5). Wang et al., in their study on 84 hospitalized patients with COVID-19 infection, showed that the levels of total TT3 and TSH were significantly lower in the patients than that in the healthy individuals, and 61.9% of patients showed thyroid function abnormalities (3). Additionally, Xu et al., in a systematic review of 11 studies and 2 995 COVID-19 patients, showed that thyroid dysfunction was associated with severe COVID-19 infection, with hypothyroidism having a greater relationship with severe COVID-19 infection (6). According to evidence, non-thyroidal illness (NTI) syndrome is the most common hormonal alteration observed in COVID-19 patients, and most changes in thyroid hormones are caused by cytokine storm (7). Most studies classified thyroid disorders during COVID-19 infection as euthyroid sick syndrome (ESS) or destructive thyroiditis (8, 9).
Although a number of studies have examined the association of COVID-19 infection with thyroid hormones, this association is not yet fully understood. Furthermore, although some studies examined the thyroid hormone in COVID-19 patients in Iran, (10, 11) the results were gathered retrospectively (10), or they evaluated the hormones once and did not check the patients after recovery (11). Therefore, the present study aimed to evaluate thyroid hormones in hospitalized patients and one month after recovery.
2. Methods
This was a comparative cross-sectional study on 78 hospitalized patients with COVID-19 infection and 80 individuals without COVID-19 infection. The design of this study was approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences Ahvaz, Iran.
2.1. Inclusion/Exclusion Criteria
Individuals with the following criteria were recruited for the study: Age up to 55 years for males and up to 45 years for females (to avoid the effect of perimenopause on thyroid hormones) and confirmed infection with COVID-19 based on polymerase chain reaction (PCR) test or lung computed tomography (CT)-scan for the groups with COVID-19 infection. Participants in the control group had never been tested for COVID-19 and did not have any clinical symptoms to make them go and get tested. Additionally, they had no history of hospitalization. Menopausal women and subjects with known hormonal dysfunction were excluded from the study. All participants provided written informed consent for participation in the study.
2.2. Measurements
A demographic questionnaire and a checklist were used to collect the data. All patients with confirmed COVID-19 infection who were hospitalized at Razi hospital of Ahvaz, Iran, were screened for eligibility. Razi hospital is a university hospital with 200 beds that was designated for the admission of patients with COVID-19 infection from the early days of the COVID-19 pandemic in Iran. The diagnosis of COVID-19 infection was made using the RT-PCR test. Individuals with positive PCR test results and symptoms that were alleviated with home remedies were hospitalized. Patients with moderate disease had blood oxygen levels higher than 93% and mild respiratory distress. A lung CT scan was requested for each patient with respiratory distress. Patients with lung infiltration higher than 50%, blood oxygen levels lower than 93%, and respiratory rates higher than 30/min were transferred to the intensive care unit (ICU). The severity of the disease was also confirmed by an infectious disease specialist.
Eligible hospitalized patients were requested to fill out a demographic questionnaire. Then, a 5 mL venous blood sample was collected from all patients, placed into gel tubes, and transferred to a reference laboratory (Novin Lab, Ahvaz, Iran). The blood samples were centrifuged for 10 minutes. Then, the sera were separated in gamma tubes with a lid and stored in a freezer (-20°C) until the time of evaluation. The measurement technique was based on quantitative luminescence, which has a much higher sensitivity and specificity than the enzyme-linked immunoassay (ELISA). The blood samples were taken by a trained midwife. One month after recovery (confirmed by negative symptoms and a negative COVID-19 test), the participants were asked to refer to the laboratory for vein puncture. The control group was apparently healthy; however, no previous thyroid test results were available for them.
Thyroid function was assessed using the measurement of TSH, total TT3, and total tetraiodothyronine (TT4). The same thyroid hormones were measured in the control group that had these tests for routine checkups (12). Data collection started in July 2020 and ended in February 2021, coincident with the second and third surges of COVID-19 in Iran (13). At this time, the vaccination of the general population had not been started yet in Iran.
The control group was recruited from individuals who were admitted to the Novin laboratory in Ahvaz for their annual checkups, and thyroid hormone measurements were among their assessments. The control group underwent thyroid tests only once at the same time that thyroid tests were measured for hospitalized patients.
2.3. Instruments
A demographic questionnaire and a checklist were used to collect the data. The demographic questionnaire included questions about age, gender, marital status, educational attainment, signs and symptoms of COVID-19, and a history of chronic diseases. The checklist was used to record the laboratory results.
2.4. Statistical Analyses
All the data were entered into SPSS software version 22 (IBM SPSS Statistics for Windows). The results were presented as mean ± standard deviation (SD) or number and frequency. The independent t-test, Chi-square test, Mann-Whitney U test, and Wilcoxon test were used to compare variables between groups. Because the age between the two groups was significant, analysis of covariance (ANCOVA) was used to adjust this effect. A P-value < 0.05 was considered statistically significant.
3. Results
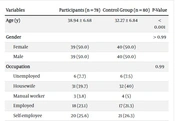
The blood samples from 78 hospitalized patients with COVID-19 infection, of whom 35 (44.8%) were in the ICU, were initially tested. One month after recovery, the patients were assessed again. The demographic characteristics of participants are shown in Table 1. As evident from Table 1, the participants in the control group were significantly younger than those in the case group (P < 0.001). The participants did not show any significant difference regarding gender, occupation, educational attainment, and marital status. Most participants in the COVID-19 group had a complex of symptoms of the infection; nevertheless, 26.9% of them had only one symptom. Chronic diseases, such as hypertension and diabetes, were present in 21 (26.9%) of the hospitalized participants.
| Variables | Participants (n = 78) | Control Group (n = 80) | P-Value |
|---|---|---|---|
| Age (y) | 38.94 ± 6.68 | 32.27 ± 6.84 | < 0.001 |
| Gender | > 0.99 | ||
| Female | 39 (50.0) | 40 (50.0) | |
| Male | 39 (50.0) | 40 (50.0) | |
| Occupation | 0.99 | ||
| Unemployed | 6 (7.7) | 6 (7.5) | |
| Housewife | 31 (39.7) | 32 (40) | |
| Manual worker | 3 (3.8) | 4 (5) | |
| Employed | 18 (23.1) | 17 (21.3) | |
| Self-employee | 20 (25.6) | 21 (26.3) | |
| Education | 0.99 | ||
| Primary | 20 (25.6) | 19 (23.8) | |
| High school | 11 (14.1) | 13 (16.3) | |
| Diploma | 27 (34.6) | 26 (32.5) | |
| University education | 20 (25.6) | 22 (27.5) | |
| Marital status | 0.91 | ||
| Single | 10 (12.8) | 12 (15) | |
| Married | 68 (87.2) | 68 (85) | |
| Chronic diseases b | - | ||
| Have not | 57 (73.1) | - | |
| Have | 21 (26.9) | - | |
| Signs and symptoms of COVID-19 infection | - | ||
| Fever + chills + shortness of breath, and pain in the body | 11 (14.1) | - | |
| Fever + shortness of breath + chills and headache | 16 (20.5) | - | |
| All symptoms | 30 (38.5) | - | |
| One of the following symptoms, such as fever, cough, headache, or shortness of breath | 21 (26.9) | - |
Abbreviation: COVID-19, coronavirus disease 2019.
a Values are presented as No. (%) or mean ± SD.
b Chronic diseases, such as hypertension and diabetes.
Table 2 shows the level of thyroid hormones in participants with COVID-19 infection and in the control group. As Table 2 shows, the level of TSH at the baseline in the hospitalized patients was significantly lower than that in the control group (P < 0.0001). The level of this hormone increased one month after recovery from the infection (P = 0.0001).
| Variables | Case (n = 78) | Control (n = 80) | P-Value b |
|---|---|---|---|
| TSH (mlU/L) | < 0.0001 | ||
| Baseline | 1.24 ± 1.08 | 2.05 ± 1.02 | |
| Follow-up | 1.87 ± 1.26 | - | |
| P-value | 0.0001 | - | |
| TT3 (ng/dL) | 0.188 | ||
| Baseline | 1.20 ± 0.24 | 1.28 ± 1.25 | |
| Follow-up | 1.00 ± 0.27 | - | |
| P-value | < 0.0001 | - | |
| TT4 (ng/dL) | 0.076 | ||
| Baseline | 8.48 ± 2.27 | 7.76 ± 1.43 | |
| Follow-up | 7.31 ± 1.83 | - | |
| P-value | < 0.001 | - |
Abbreviations: TSH, thyroid-stimulating hormone; TT3, triiodothyronine; TT4, tetraiodothyronine.
a Values are presented as mean ± SD.
b Analysis of covariance (ANCOVA) test was used, adjusted for age.
The mean level of TT3 did not show statistically significant at the baseline but decreased after recovery from the infection (P < 0.0001). The level of TT4 in the case group was high at the baseline in comparison to the control group (8.48 ± 2.27 vs. 7.76 ± 1.43, P = 0.076) that was reduced in the follow-up period (7.31 ± 1.83, P < 0.001).
Table 3 was designed to show the differences between the two groups of patients with moderate and severe infection. As Table 3 shows, at the baseline, the mean of TSH was non-significantly lower in the group with severe infection. The increase in the level of TSH in the group with severe disease was more dominant in the follow-up period. The levels of TT3 and TT4 at the baseline did not show any significant difference; nevertheless, in the follow-up, the mean of TT4 decreased more than that of the patients with moderate disease (P = 0.014).
| Variables and Outcomes | Disease Severity | P-Value | |
|---|---|---|---|
| Moderate (n = 43) | Severe (n = 35) | ||
| TSH (mlU/L) | |||
| Baseline (n = 78) | 1.42 ± 1.22 | 1.02 ± 0.83 | 0.27 b |
| Follow-up (n = 69) | 1.55 ± 1.06 | 2.27 ± 1.38 | 0.024 b |
| TT3 (ng/dL) | |||
| Baseline (n = 78) | 1.19 ± 0.26 | 1.20 ± 0.21 | 0.513 b |
| Follow-up (n = 69) | 1.04 ± 0.25 | 0.95 ± 0.29 | 0.146 b |
| TT4 (ng/dL) | |||
| Baseline (n = 78) | 8.48 ± 2.46 | 8.48 ± 2.04 | 0.99 c |
| Follow-up (n = 69) | 7.80 ± 1.65 | 6.72 ± 1.90 | 0.014 c |
Abbreviations: TSH, thyroid-stimulating hormone; TT3, triiodothyronine; TT4, tetraiodothyronine
a Values are presented as mean ± SD.
b Mann-Whitney U test.
c Independent t-test.
4. Discussion
This study was designed to evaluate the levels of thyroid hormones in COVID-19 patients during the active period of the disease and one month after recovery. The results of the present study showed that the levels of TSH in patients decreased during the active period of COVID-19 infection in comparison to the control group; however, in the follow-up period, an increase in the level of this hormone was observed. Additionally, the level of TT3 was slightly reduced, and the level of TT4 was high during the active period of infection, which fell significantly in the follow-up period.
In line with the results of the current study, Wang et al. studied 84 hospitalized COVID-19 patients and showed that the levels of TT3 and TSH in these patients were lower than in healthy individuals (P < 0.001). Wang et al. concluded that abnormalities with thyroid hormones mainly result from NTI syndrome and not true thyroid abnormalities. The results of the present study are in line with Wang et al.’s results, as all abnormalities in thyroid hormones returned to normal after recovery from infection (3). Additionally, similar to the results of the current, Weiwei et al., in their study on 1 395 individuals, demonstrated that the level of free thyroxine (FT4) increased, and the levels of TSH and free triiodothyronine (FT3) decreased significantly in patients with COVID-19 infection (14).
Razu et al., in a study on 30 confirmed cases of COVID-19 infection who had not been already vaccinated, observed that the levels of both TT3 and TT4 decreased; nevertheless, the former underwent a more pronounced reduction than the latter (15). Unlike the results of Razu et al.'s study, the results of the present study showed a moderately slight decrease in the level of TT3 and a non-significant increase in the level of TT4, compared to the control group. This discrepancy might be related to the fact that Razu et al. assessed only 10 patients in the group of unvaccinated COVID-19 infection, or it could be due to the fact that in the current study, the patients underwent corticosteroid therapy upon admission to the hospital, which might have caused these manifestations (15).
The mechanism of change in thyroid hormones in infectious diseases is unknown. Studies have shown that the cause of changes in the level of thyroid hormones, especially TSH, during infectious diseases, might not relate to the pituitary origin and might result from outside of the pituitary gland, such as bone marrow (16). At the present time, there is no evidence in favor of the direct thyroid effect of cytokines on thyroid hormones (7). Other studies explained the role of angiotensin-converting enzyme 2 (ACE2) receptors in the entry of viruses into the thyroid cells and cytokine storm (17). The thyroid gland is reach of the ACE2 receptor that permits the severe acute respiratory syndrome coronavirus 2 (SAR-CoV-2) viruses to enter this gland easily (18). Additionally, apoptosis might play a role in the thyroid dysfunction associated with severe acute respiratory syndrome (SARS) disease (19).
In the present study, 35 patients (44.8%) had a severe form of the disease and were admitted to ICU, and none of them died. The results of the present study showed that the level of TSH was lower in patients with severe disease at the baseline and in the follow-up period and that the level of TT3 at baseline and in the follow-up period was not different between the groups with severe and moderate disease. The level of TT4 was not different at the baseline but was significantly lower in the follow-up period in the group with severe disease.
In a study on hospitalized patients affected with COVID-19 infection, Muller et al. observed that patients with more severe disease showed thyrotoxicosis and low levels of TSH (20). Chen et al. conducted a retrospective study on 50 patients and observed that lower TSH was present in 56% of COVID-19 patients, and their levels of TT3 were lower than in healthy individuals. They concluded that thyroid hormone levels were more likely to change in individuals with severe COVID-19 disease (2). Additionally, Beltrao et al. reported that FT3 levels were lower in patients with severe disease; however, the serum reverse triiodothyronine (rT3) was elevated in patients with severe disease. Beltrao et al. drew the conclusion that thyroid hormones have a correlation with the severity of the disease (21).
The results of the present study are in line with the results of Muller et al., Chen et al., and Beltrao et al., except for the fact that in the present study, the alteration of thyroid hormones in patients with severe disease was not significant in comparison to patients with moderate disease. This discrepancy is attributed to the small sample size of the group with severe disease and to the corticosteroid therapy that all patients with moderate and severe disease received. Additionally, Dincer Yazan et al. showed that there is a relationship between the changes in thyroid hormones and mortality in patients with COVID-19 infection in the way that the patients with higher reduction in TSH and FT3 and higher levels of FT4 were more likely to be admitted to the ICU or die (22), which is not consistent with the results of the present study.
4.1. Limitations of the Study
This is the first study in Iran to prospectively follow up on hospitalized patients with COVID-19 infection one month after recovery from the disease. Despite its strength, this study has some limitations. Firstly, the study did not examine the level of anti-thyroid antibodies, which might have affected the obtained results. Secondly, steroid therapy regularly starts for any COVID-19 patient admitted to a hospital in Iran; therefore, the changes in thyroid hormones might be partly due to steroid therapy and not COVID-19 infection. Thirdly, this study did not measure cytokine levels in the patient group with severe COVID-19 to identify a potential link between thyroid hormone levels and cytokine storm. Finally, due to the small sample size, it is not possible to draw definitive conclusions from this study.
4.2. Conclusions
According to the results of this study, patients with COVID-19 infection showed abnormalities in thyroid hormones, such as decreased levels of TSH, which returned to normal ranges after recovery. Patients with severe COVID-19 showed lower levels of TSH and unchanged levels of TT3 and TT4 in comparison to the patients with moderate disease. Further investigations on thyroid function in patients with COVID-19 are recommended.