1. Background
In recent years, chronic kidney disease (CKD) has emerged as a significant public health concern, with potential consequences such as increased susceptibility to end-stage kidney disease (ESKD), which necessitates interventions like dialysis and kidney transplantation (1). Nephropathy, a microvascular complication of hyperglycemia, is the most common cause of CKD and one of the most prevalent complications of diabetes mellitus (DM). Approximately 40% of diagnosed CKD cases and 40 - 55% of ESKD cases in the United States are directly attributed to nephropathy. Studies indicate that around 30% of patients with type 1 DM and a smaller proportion of those with type 2 diabetes progress to ESKD. However, due to the higher prevalence of type 2 diabetes, the total number of ESKD cases related to type 2 diabetes is greater (2).
Diabetic nephropathy is diagnosed through increased urinary albumin, decreased glomerular filtration rate (GFR), and histopathological findings. Various factors associated with diabetic nephropathy include smoking, obesity, race, ethnicity, dyslipidemia, hypertension, genetic factors, and family history (FH) of diabetes mellitus (3). Studies have identified a positive FH of DM as an independent risk factor for diabetic nephropathy (4, 5), and FH of DM is positively correlated with gene polymorphisms linked to diabetic nephropathy (6).
The primary pathogenesis of nephropathy involves elevated blood glucose levels. Individuals with a hemoglobin A1C (HbA1c) above 8% have a twofold risk of developing nephropathy compared to those with an HbA1c below 6% (7, 8). This suggests that while hyperglycemia in diabetes mellitus is associated with an increased risk of nephropathy, patients with non-diabetic hyperglycemia, such as prediabetes, are also at risk. Up to one-third of adults newly diagnosed with diabetic nephropathy show some degree of renal impairment before meeting the diagnostic threshold for diabetes mellitus based on blood glucose levels. Consequently, the adverse effects of hyperglycemia on the kidneys begin at the prediabetic stage. Studies have shown that prediabetic hyperglycemia has detrimental effects on the kidneys and is associated with both nephropathy and CKD (9).
Previous studies have identified elevated glomerular filtration rate as an early indicator of potential kidney damage. This occurs due to increased glomerular pressure and plasma flow, which ultimately leads to albuminuria (10). Diabetic hyperglycemia is a known contributor to elevated GFR. Hyperglycemia resulting from diabetes mellitus increases oxidants and causes subsequent inflammation. This inflammation enhances the permeability of blood vessels, leading to albuminuria (11). Since the increase in GFR and albuminuria in nephropathy is associated with hyperglycemia, it is plausible to hypothesize that elevated blood glucose levels during the prediabetic stages may be linked to nephropathy (12).
In recent years, the incidence and prevalence of prediabetes have been rising alongside diabetes, causing widespread concern among global health systems. The World Health Organization estimates that over 470 million individuals worldwide will have prediabetes by 2030 (13). Effective management of nephropathy requires identifying patients at higher risk for developing this condition.
2. Objectives
Given that a positive family history (FH) of DM in prediabetic patients may be associated with an increased risk of nephropathy, the present study aimed to investigate the incidence of nephropathy and associated factors, such as a positive FH of DM in first-degree relatives and laboratory findings in prediabetic patients.
3. Methods
3.1. Study Design and Patients
This retrospective study, conducted in 2021, focused on outpatients referred to the Isfahan Endocrine and Metabolism Research Center in 2004 and followed up at least until 2019. Patients included in the study had prediabetes and no evidence of urinary tract infection, hematuria, fever, uncontrolled hypertension, pregnancy, heart failure (with an ejection fraction of less than 40% in initial examinations), or other acute and chronic diseases that could lead to proteinuria, kidney failure, or transient hyperglycemia based on laboratory and clinical findings from 2004. Patients who progressed to diabetes or showed evidence of urinary tract infection, hematuria, fever, uncontrolled hypertension, pregnancy, heart failure, or other conditions that could lead to proteinuria, kidney failure, or transient hyperglycemia in the 2019 medical records were excluded.
Out of 3,153 medical records from 2004, 1,230 patients were enrolled based on the inclusion and exclusion criteria. Of these, 411 (33.4%) patients had missing medical records in 2019 and were excluded, leaving 819 patients (66.6%) for analysis.
3.2. Ethical Considerations
The present study was approved by the Isfahan University of Medical Sciences Research Ethics Committee (Ref. ID: IR.MUI.MED.REC.1400.221) and was conducted in accordance with the ethical standards outlined in the 1964 Declaration of Helsinki and its later amendments, or comparable ethical standards. This study was part of the Internal Medicine Specialist Degree thesis of S. A. and F. A. at the university (Thesis number: 53546).
3.3. Data Collection and Variables
Using data from the Isfahan Diabetes Presentation Study, we collected information from 2004 and 2019, including demographic variables, family history of diabetes mellitus, blood pressure levels (mmHg), and baseline laboratory findings. These findings included lipid profile evaluations (low-density lipoprotein [LDL], high-density lipoprotein [HDL], triglycerides [TG]), fasting blood sugar (FBS), postprandial blood glucose at 30, 60, and 120 minutes after test meals, random urine albumin and creatinine levels, and serum creatinine levels. This comprehensive data collection was carried out by two co-authors using a meticulously prepared checklist developed by the project team.
Blood pressure measurements for patients during physician visits at the study center were typically taken using handheld or standard mercury sphygmomanometers from reputable brands such as Reister and Zenith Med. These measurements were conducted by trained technicians and physicians.
Laboratory assessments for the patients were generally performed at the Isfahan Endocrine and Metabolism Research Center laboratory. Serum creatinine levels were measured using the Biorex kit, with normal ranges set at 0.6 to 1.3 mg/dL for women and 0.7 to 1.4 mg/dL for men. Blood sugar levels were assessed using the BIONIK kit, with normal levels ranging from 70 to 115 mg/dL based on age. Low-density lipoprotein measurements were conducted with the BIONIK kit, where values below 100 mg/dL were considered normal, and levels above 160 mg/dL were deemed high. TG levels were measured using the BIONIK kit, with normal values below 150 mg/dL, borderline values ranging from 150 to 199 mg/dL, high values ranging from 200 to 499 mg/dL, and very high values exceeding 500 mg/dL. High-density lipoprotein levels were measured with the Ziest Chem kit, with normal values above 30 mg/dL.
Patients were divided into two groups: Those with a FH of DM and those without. The incidence of nephropathy over 15 years was compared between these two groups. Possible factors affecting the incidence of nephropathy were also compared between patients with and without nephropathy based on the 2019 findings.
3.4. Diagnosis of Prediabetes and Diabetes
Prediabetes was diagnosed based on a fasting blood sugar (FBS) level of 100 - 125 mg/dL, a two-hour postprandial plasma glucose level of 140 - 199 mg/dL, or an HbA1c level of 5.7-6.4% (39 - 46 mmol/mol). Diabetes was diagnosed with an FBS level of 126 mg/dL or higher, a two-hour postprandial plasma glucose level of 200 mg/dL or higher, or an HbA1c level of 6.5% or higher (46 mmol/mol or higher) (14). Most patients had their blood sugar measured using the enzymatic method with a Pars test kit and Coba’s analyzer. Hemoglobin A1C levels were determined using chromatography with a Pars test kit and Cobas autoanalyzer at the Isfahan Endocrine and Metabolism Research Center laboratory.
3.5. Diagnosing Nephropathy and Assessing Estimated GFR (eGFR)
The random urine albumin-to-creatinine ratio (ACR) ≥ 30 mg/g was used as a criterion for diagnosing nephropathy. Additionally, albuminuria of 30 - 300 mg/g lasting more than 3 months indicates chronic kidney disease (15). The glomerular filtration rate for the studied patients was estimated using serum creatinine levels, age, sex, and body weight, following the Cockcroft-Gault equation. Microalbumin levels in most patients were assessed using the turbidimetric method with a Pars test kit and Cobas analyzer at the Isfahan Endocrine and Metabolism Research Center laboratory.
3.6. Statistical Analysis
Data were analyzed using IBM SPSS Statistics (Version 27). Frequencies and percentages were used to present qualitative data, while quantitative data were expressed as means and standard deviations (SD). For inferential analysis, an independent t-test was employed to compare the means of quantitative variables between patients with and without nephropathy. Univariate and multivariate logistic regression analyses were conducted to identify factors affecting the incidence of diabetic nephropathy. A P-value of less than 0.05 was considered statistically significant.
4. Results
Of the 3,153 patients examined, 1,616 (51.2%) had no blood glucose disorders, 307 (9.7%) had diabetes, and 1,230 (39%) had prediabetes. The mean (SD) age of patients with prediabetes was 44.0 (6.8) years, and 906 (73.7%) of them were women. At baseline, 303 (24.6%, 95% CI: 22.2%, 27.1%) patients were diagnosed with nephropathy.
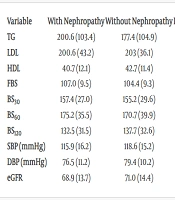
Table 1 presents laboratory and clinical findings theoretically related to nephropathy. No significant difference was found between the means of nephropathy-related factors in patients with and without nephropathy (P ≥ 0.05).
| Variables | With Nephropathy | Without Nephropathy | P-Value a |
|---|---|---|---|
| TG | 200.6 (103.4) | 177.4 (104.9) | 0.142 |
| LDL | 200.6 (43.2) | 203 (36.1) | 0.670 |
| HDL | 40.7 (12.1) | 42.7 (11.4) | 0.265 |
| FBS | 107.0 (9.5) | 104.4 (9.3) | 0.062 |
| BS30 | 157.4 (27.0) | 155.2 (29.6) | 0.647 |
| BS60 | 175.2 (35.5) | 170.7 (39.9) | 0.465 |
| BS120 | 132.5 (31.5) | 137.7 (32.6) | 0.291 |
| SBP (mmHg) | 115.9 (16.2) | 118.6 (15.2) | 0.236 |
| DBP (mmHg) | 76.5 (11.2) | 79.4 (10.2) | 0.062 |
| eGFR | 68.9 (13.7) | 71.0 (14.4) | 0.390 |
Abbreviations: TG, Triglycerides; LDL, Low-Density Lipoproteins; HDL, high-density lipoproteins; FBS, fasting blood sugar; BS30; 60; 120, postprandial blood glucose at 30; 60; and 120 minutes after test meals; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate.
a Independent sample t-test.
Fifteen years after the baseline, data were available for 819 patients (66.6%), of whom 496 (60.6%, 95% CI: 57.1%, 63.9%) had prediabetic nephropathy. The prevalence of nephropathy increased from 24.6% (95% CI: 22.2%, 27.1%) to 60.6% (95% CI: 57.1%, 63.9%) over 15 years. The 15-year cumulative incidence of nephropathy was calculated to be 36% (95% CI: 32.9%, 38.9%), and the mean annual incidence was 2.4% (95% CI: 1.6%, 3.6%).
To investigate the association between a positive family history of diabetes mellitus (DM) and nephropathy, the best model was obtained using multivariate logistic regression analysis. This analysis accounted for the confounding effects of sex, age, fasting blood sugar, HbA1c, smoking, and Body Mass Index (BMI). The results showed no significant relationship between a positive FH of DM and the incidence of nephropathy (P = 0.638). However, male sex (P = 0.001), age (P = 0.023), and BMI (P = 0.024) were significantly associated with nephropathy (Table 2).
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| Odds Ratio | P-Value | Odds Ratio | P-Value | |
| Positive family history of diabetes mellitus | 0.890 | 0.241 | 0.722 | 0.638 |
| Age | - | - | 1.027 | 0.023 |
| Sex (male) | - | - | 1.816 | 0.001 |
| HbA1C | - | - | 1.171 | 0.100 |
| BMI | - | - | 1.050 | 0.014 |
Abbreviation: BMI, Body Mass Index; HbA1c, hemoglobin A1C.
5. Discussion
In our study, the prevalence of nephropathy increased from 24.6% at baseline to 60.6% after 15 years. The 15-year cumulative incidence of nephropathy was 36%, and the mean annual incidence was 2.4%. Previous studies have reported the annual incidence of nephropathy in people with type 2 diabetes to be approximately 3.6% (16) and in patients with prediabetes to be around 1.7% (17), which aligns with our findings. The prevalence of nephropathy in prediabetes patients ranges between 4.5% and 26% (18), while the prevalence of diabetic nephropathy in the United States is approximately 25% (19), which is close to the 39% prevalence observed at baseline in our study. The higher prevalence of nephropathy observed in our prediabetes patients may be attributed to the study site selection. It is possible that most patients referred to the treatment center were at more advanced stages of the disease and had developed complications, representing a more severe patient population compared to those in other settings.
In assessing factors theoretically related to nephropathy, we observed that mean levels of triglycerides (TG), low-density lipoprotein, and fasting blood sugar were higher in the nephropathy group, while mean levels of high-density lipoprotein, blood pressure, and estimated glomerular filtration rate were higher in patients without nephropathy. However, these differences were not statistically significant. Previous studies have shown associations between higher TG, LDL, blood sugar, and blood pressure, and lower HDL and eGFR with nephropathy (11, 20, 21). This discrepancy may be due to several factors. Firstly, the differences in our study were not statistically significant. Additionally, this study did not account for the use of antihypertensive medications in the patients examined. Given that higher age and BMI were associated with nephropathy in our study and such patients likely receive better medical care, it is possible that hypertension in these patients is managed more effectively, leading to lower blood pressure levels compared to patients without nephropathy.
Regarding our findings on male sex, HbA1c, age, and BMI being positively associated with nephropathy incidence, other studies have also identified these variables as risk factors for nephropathy in patients with hyperglycemia (5, 22, 23). Hu and Zhang found that patients with elevated HbA1c had a 1.35-fold increased risk of nephropathy (5). Lou et al. reported that elevated HbA1c and higher-than-normal BMI had odds ratios of 1.57 and 1.65, respectively, for developing nephropathy (24). Similarly, Shahwan et al. found that male sex, older age, higher HbA1c, and BMI were associated with nephropathy in a study of 550 patients over 35 years of age with type 2 diabetes (25).
In our study, no significant relationship was observed between a positive family history of diabetes mellitus and the incidence of nephropathy. Research has explored potential genetic backgrounds of nephropathy by examining familial clustering and racial/ethnic differences among patients with nephropathy (26-28). However, some studies have also found no significant association between FH of DM and nephropathy, consistent with our results (5). Since not all but approximately 70% of patients with prediabetes progress to diabetes within 10 years (29), the association between a positive FH of DM and nephropathy might be lower in prediabetes patients compared to those with diabetes. Further research is needed in this area to explore these relationships more comprehensively.
5.1. Limitations
This study had limitations. It was conducted at a single center, which may limit the generalizability of the findings. Multicenter studies would be beneficial for obtaining more representative results. Additionally, as a retrospective study, it could not investigate certain factors that might influence the long-term incidence of nephropathy. For instance, we were unable to assess the intensity of blood glucose management and blood pressure control during the study period, which could have provided further insights into nephropathy development in our patient population.
Despite these limitations, the study had notable strengths. It investigated both the incidence and prevalence of nephropathy, which is not typically possible in cross-sectional studies. The study also considered various confounding variables in analyzing factors associated with nephropathy. Conducted at one of the largest endocrinology research centers in Iran, it provides valuable data that can be generalized to similar populations.
5.2. Conclusion
In this study, male sex, older age, and BMI were found to have a significant positive association with the incidence of nephropathy, while no significant relationship was observed between a positive FH of DM and nephropathy. These findings suggest that factors such as male sex, age, and BMI play a more significant role in the development of nephropathy in this population. Therefore, these factors should be considered in risk assessment and management strategies for preventing nephropathy in patients with prediabetes. Further research is needed to explore additional potential risk factors and to develop effective preventive measures, which could lead to improved patient outcomes and a reduction in the prevalence of chronic kidney disease caused by nephropathy. Investigating the role of the duration of prediabetes in nephropathy development is also recommended for future studies.