1. Background
Environmental and lifestyle factors contribute to 90 - 95% of cancers (1). Like the skin and lungs, the bladder is an organ that constantly interacts with the environment, making it susceptible to environmental carcinogens. The two most common sources of environmental exposure are tobacco smoking and occupational hazards. The primary known cause of bladder cancer (BC) is tobacco, responsible for 30% to 40% of urothelial carcinoma cases. The risk of developing BC is about 2 to 3 times greater in smokers (2, 3). Occupational exposures account for 5% to 10% of all BC cases, with occupations involving direct contact with chemicals and dyes having the highest lifetime risk (2, 4). Aromatic amines are known carcinogenic substances associated with an elevated risk of BC (4-6).
It has long been believed that the incidence of BC is much more common in men than in women (7, 8). Occupations particularly at risk for exposure to aromatic amines include those in the tobacco, dye, and rubber industries, as well as hairdressers, painters, and leather workers. Additionally, those working with polycyclic aromatic hydrocarbons, such as nurses, waiters, aluminum workers, and petroleum workers, are also at risk (9-11). Many of these occupations are increasingly populated by women, and it has been reported that occupational exposure may be particularly increasing among women (12, 13).
The epidemiology of occupational cancers in Iran is still largely unknown, and there is no robust and sufficient evidence of occupational cancer risks. Furthermore, the risks associated with many occupations, such as agriculture, remain unclear. Despite the indiscriminate use of pesticides and other chemicals in Iran, agriculture is rarely considered a high-risk occupation.
2. Objectives
Considering that the geographical distribution of cancer varies due to environmental factors and that no study has been conducted on this subject in Guilan Province, this study aimed to assess the exposure to occupational carcinogens and the risk of BC in Guilan Province in northern Iran.
3. Methods
This case-control study was conducted among 457 participants, including 266 BC patients and 191 individuals in the control group. All participants were adults aged 18 years and older from Guilan Province.
The case population included all diagnosed BC patients in Guilan Province who were registered at the Guilan Cancer Registry in the Vice-Chancellor of Health, Guilan University of Medical Sciences, under the name of Sima-e-Sartan. Non-urothelial BC cases were excluded from the study. Controls were recruited from attendees of an occupational health clinic and accompanying individuals at Razi University Hospital, provided they had no history of cancer or underlying diseases.
We interviewed all controls and used the records of the case population to collect data. In cases where data were missing from the case group, information was gathered through phone interviews. The census method was used to collect data within the relevant time interval. The information gathered included demographic characteristics, current and previous residence, addiction status, water consumption habits, current and previous occupation, environmental exposures and the duration of each, cancer history, treatment outcomes, and family cancer history. History of exposure to chemical compounds such as aromatic amines, arsenic, creosote, tar and carbon derivatives, combustion and greenhouse gases, gasoline, amino biphenyl, chloronfazine, methylene bis/chloroaniline, aluminum, herbicide/pesticide, aromatic hydrocarbons, rubber production, ammonium nitrate, lead, mustard, talc, chalk, etc., for each participant was collected.
The study rigorously followed ethical principles in human research. Ethical clearance was granted by the Research Ethics Committee of Guilan University of Medical Sciences (Code: IR.GUMS.REC.1400.196) prior to data collection. Participants provided informed consent, fully comprehending the study's objectives and procedures. Absolute confidentiality of personal information was ensured, with paramount importance given to participant safeguarding at every stage.
3.1. Statistical Analysis
Data analysis was conducted using SPSS software version 24. Descriptive statistics were used for quantitative data, and frequencies and percentages were calculated for qualitative variables. Chi-Square and Fisher's exact tests were used to compare occupational frequencies between groups, while the odds ratio measured the association between occupation and BC. Multiple logistic regression (LR) models were employed to assess this relationship, considering confounding variables. The model's goodness-of-fit and predictive ability were evaluated using the Hosmer-Lemeshow test and Nagelkerke R², respectively. Statistical significance was set at P < 0.05 for all tests.
4. Results
The results show significant differences between the two groups in terms of individual characteristics (gender, age, marital status, etc.) (all with P < 0.01). The type of water consumed had a significant effect on the occurrence of BC (P < 0.001). Regarding lifestyle factors, there was a significant association between cigarette consumption and BC (P = 0.0001). The duration of smoking (years), the number of cigarettes smoked per day, and opium consumption also showed significant differences between the groups (P = 0.004, 0.001, and 0.001, respectively). However, there was no significant relationship between alcohol consumption and BC (P = 0.784). Place of residence (current and previous) also showed significant associations with BC (P = 0.0001) (Table 1).
| Variables | Cases | Controls | P-Value |
|---|---|---|---|
| Gender | 0.009 | ||
| Male | 224 (84.2) | 142 (74.3) | |
| Female | 42 (15.8) | 49 (25.7) | |
| Age group (y) | 0.0001 | ||
| Less than 50 | 15 (5.6) | 42 (22) | |
| 51 - 60 | 45 (16.9) | 62 (32.5) | |
| 60 - 70 | 82 (30.8) | 67 (35.1) | |
| More than 70 | 124 (46.6) | 20 (10.5) | |
| Age (y) | 67.55 ± 12.38 | 56.43 ± 13.29 | 0.0001 |
| Marital status | 0.001 | ||
| Married | 196 (73.7) | 159 (83.2) | |
| Single | 18 (6.8) | 19 (9.9) | |
| Divorced | 7 (2.6) | 1 (0.5) | |
| Widowed | 45 (16.9) | 12 (6.3) | |
| Current residence | 0.0001 | ||
| Urban | 158 (59.4) | 177 (92.7) | |
| Rural | 108 (40.6) | 14 (7.3) | |
| Years of current residence | 23.14 ± 54.89 | 18.95 ± 47.59 | 0.0001 |
| Previous residence | 0.0001 | ||
| Urban | 121 (45.5) | 144 (75.4) | |
| Rural | 145 (54.5) | 47 (24.6) | |
| Years of previous residence | 55.22 ± 20.74 | 21.16 ± 43.65 | 0.0001 |
| Type of drinking water | 0.0001 | ||
| Piping | 196 (73.7) | 176 (92.1) | |
| Well water | 65 (24.4) | 6 (3.1) | |
| etc. | 5 (1.9) | 9 (4.7) | |
| Family history of bladder cancer | 0.378 | ||
| Yes | 19 (7.1) | 18 (9.4) | |
| No | 247 (92.9) | 173 (90.6) | |
| Current employment status | 0.0001 | ||
| Unemployed | 4 (1.5) | 2 (1) | |
| Employed | 123 (46.2) | 163 (85.3) | |
| Disabled | 46 (17.3) | 2 (1) | |
| Retired | 93 (35) | 24 (12.6) | |
| Shift work | 0.001 | ||
| Work morning | 15 (10.9) | 48 (28.7) | |
| Work evening | 1 (0.7) | 3 (1.8) | |
| Night work | 1 (0.7) | 0 (0) | |
| Rotational | 121 (87.7) | 116 (69.5) | |
| Cigarettes smoking | 0.0001 | ||
| Yes | 137 (51.5) | 54 (28.3) | |
| No | 129 (48.5) | 137 (71.7) | |
| Years of smoking | 33.95 ± 15.09 | 26.07 ± 20.37 | 0.004 |
| Number of cigarettes smoked per day | 16.78±8.93 | 12.88±10.07 | 0.001 |
| Alcohol consumption (y) | 28.1 ± 16.74 | 27.83 ± 16.77 | 0.961 |
| Opium consumption | 0.001 | ||
| Yes | 55 (20.7) | 9 (6.3) | |
| No | 211 (79.3) | 179 (93.7) | |
| Opium consumption (y) | 16.76 ± 12.07 | 23.25 ± 15.42 | 0.0158 |
| Use of synthetic drugs | 0.87 | ||
| Yes | 108 (40.6) | 14 (7.3) | |
| No | 158 (59.4) | 177 (92.7) | |
| Use of synthetic drugs (y) | 12.00 ± 15.81 | 9.75 ± 13.57 | 0.828 |
| Comorbidities | 0.0001 | ||
| No | 155 (58.3) | 145 (75.9) | |
| Genitalia | 57 (21.4) | 9 (4.7) | |
| Recurrent infection | 8 (3.0) | 0 (0.0) | |
| Others | 46 (17.3) | 37 (19.4) |
Characteristics of the Study Participants a
We identified a noteworthy association between occupational categories among participants in both groups (P = 0.0001). A significant difference in employment duration (in years) was observed in patients with BC (P < 0.001).
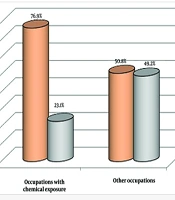
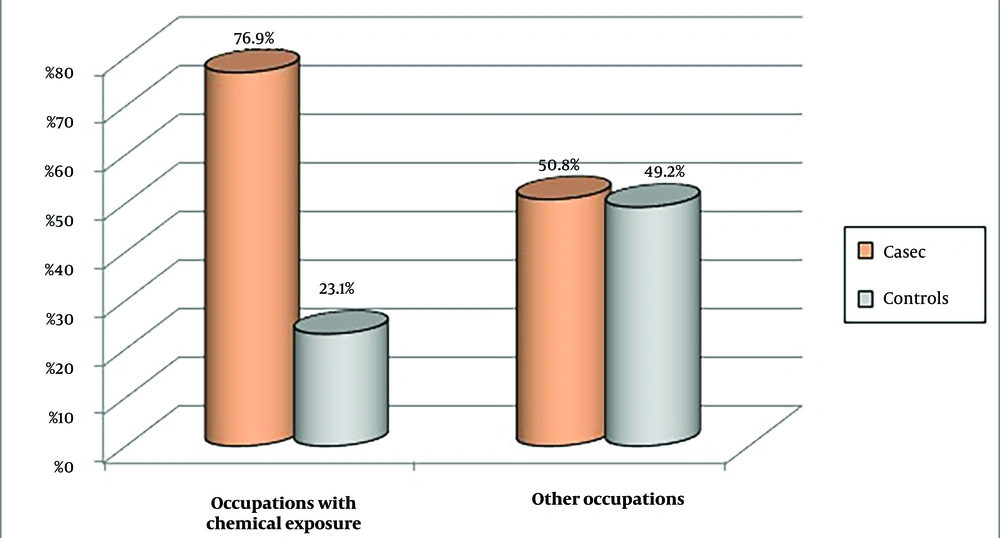
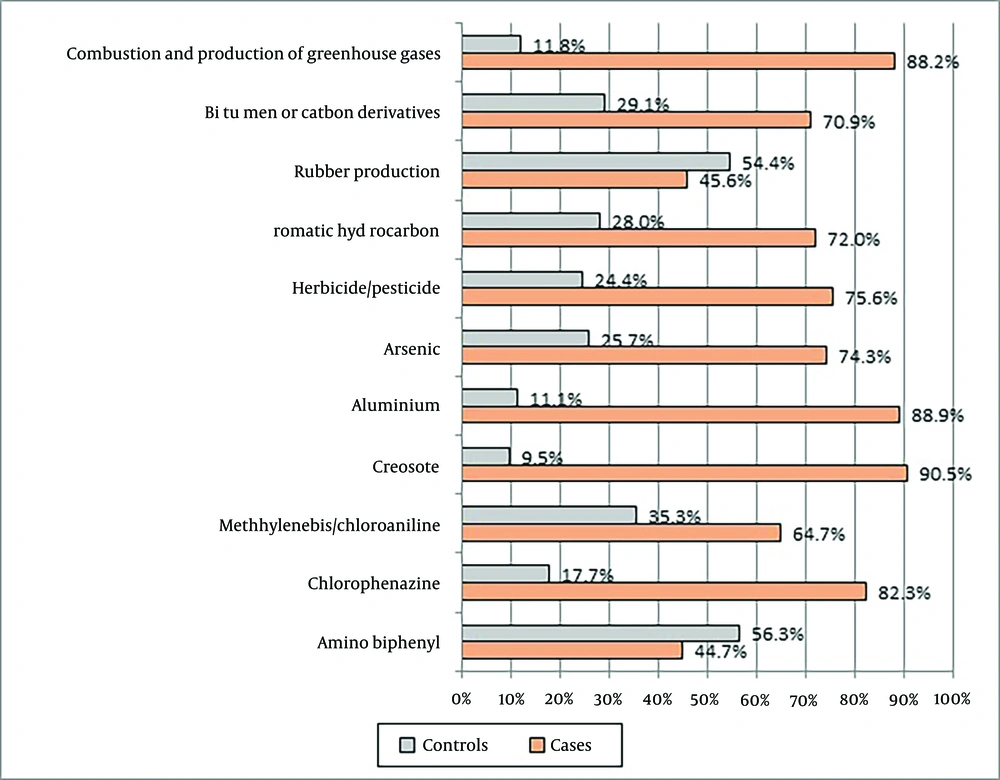
The results revealed a significant difference between healthy individuals and those diagnosed with BC in terms of chemical-related occupations (P < 0.001). Of the 47 substances analyzed for occupational exposure in our study, several showed a significant positive association with the risk of developing BC (Figures 1 and 2).
Using logistic regression with the Enter method, our study found significant associations between various intervention variables and BC incidence. Age groups of 60 - 70 years and above 70 years exhibited a 2.73-fold and 17.1-fold increased risk of BC, respectively. Living in rural areas showed a 4.76-fold higher risk, and consuming well water showed a 7-fold increased risk. Smoking and regular opium consumption were associated with a 1.81-fold and 2.97-fold higher likelihood of BC, respectively. Additionally, a history of genitourinary system diseases increased the risk by 4.54-fold. Moreover, exposure to tar and carbon derivatives increased the risk by 2.99-fold, while exposure to combustion and greenhouse gases showed a 1.12-fold increased risk of BC.
In a comprehensive six-step analysis using logistic regression with the Backward (LR) Stepwise method, we assessed the impact of intervention variables (P < 0.1) on BC incidence. Age groups of 60 - 70 years and above 70 years showed 2.80-fold and 18.2-fold increased risks, respectively, of developing BC. Residing in rural areas, compared to urban areas, was associated with a 5.06-fold higher risk. Additionally, consuming well water, smoking, and regular opium consumption (more than once a week) were found to be linked to increased probabilities of BC by 6.12-fold, 2.03-fold, and 2.58-fold, respectively. Moreover, a history of genitourinary system diseases showed a 4.35-fold increased risk of BC (Table 2).
| Variables | B | S.E. | Wald | Sig. | Exp (B) | 95.0% C.I.for EXP (B) | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Step 1 | |||||||
| Aged 61 - 70 (y) | 1.005 | 0.443 | 5.160 | 0.023 | 2.733 | 1.148 | 6.507 |
| Age over 70 (y) | 2.843 | 0.503 | 31.999 | 0.000 | 17.172 | 6.412 | 45.989 |
| Living in the rural areas | 1.561 | 0.367 | 18.077 | 0.000 | 4.763 | 2.320 | 9.782 |
| Well water | 1.947 | 0.994 | 3.838 | 0.050 | 7.005 | 0.999 | 49.107 |
| Smoking | 0.594 | 0.301 | 3.901 | 0.048 | 1.811 | 1.005 | 3.265 |
| Opium use more than once a week | 1.091 | 0.550 | 3.942 | 0.047 | 2.978 | 1.014 | 8.743 |
| Genitourinary system | 1.514 | 0.460 | 10.832 | 0.001 | 4.543 | 1.845 | 11.191 |
| Step 6 | |||||||
| Aged 61 - 70 (y) | 1.030 | 0.406 | 6.428 | 0.011 | 2.802 | 1.263 | 6.214 |
| Age over 70 (y) | 2.906 | 0.468 | 38.622 | 0.000 | 18.276 | 7.310 | 45.692 |
| Living in the rural areas | 1.623 | 0.362 | 20.117 | 0.000 | 5.066 | 2.493 | 10.294 |
| Well water | 1.813 | 0.966 | 3.519 | 0.041 | 6.126 | 0.922 | 40.705 |
| Smoking | 0.709 | 0.283 | 6.274 | 0.012 | 2.032 | 1.167 | 3.539 |
| Opium use more than once a week | 0.950 | 0.505 | 3.533 | 0.049 | 2.585 | 0.960 | 6.962 |
| Genitourinary system | 1.471 | 0.452 | 10.593 | 0.001 | 4.352 | 1.795 | 10.551 |
Logistic Regression with Backward Stepwise Method to Investigate the Effect of Intervening Variables on Bladder Cancer Incidence
In a four-step analysis using logistic regression with the Forward LR Stepwise method, we investigated the impact of intervention variables (P < 0.1) on BC incidence. The final model revealed that exposure to tar and carbon derivatives increased the likelihood of developing BC by 3.53-fold, while exposure to combustion and greenhouse gases was associated with a 10.72-fold increased risk of BC (Table 3).
| Variables and Step 4 | B | S.E. | Wald | Sig. | Exp (B) | 95.0% C.I. for EXP (B) | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Tar and carbon derivatives | 1.262 | .234 | 29.131 | 0.000 | 3.532 | 2.234 | 5.585 |
| Combustion and greenhouse gases | 2.373 | .338 | 49.183 | 0.000 | 10.725 | 5.526 | 20.814 |
Logistic Regression with Forward Stepwise Method to Investigate the Effect of Intervening Variables on Bladder Cancer Incidence
In total, the results showed that workers in the occupations of agriculture, housekeeping, and office clerks in Guilan Province are at a higher risk of contracting BC.
5. Discussion
Only 5 to 10 percent of cancers are attributed to genetic issues, while a substantial proportion, around 90 to 95 percent, is linked to environmental factors and individuals' lifestyle choices (1). Past publications have estimated that 2% to 8% of total cancer cases are attributable to occupational exposures (14).
The results of our study showed that the risk of developing BC increases significantly with age. BC incidence is strongly related to age, with older age being associated with poorer outcomes. In previous studies, consistent with our findings, the highest frequency of BC was observed in the age group of 60 - 80 years (15-17).
In the present study, the likelihood of BC incidence was found to be higher among rural residents compared to urban dwellers. It is worth mentioning that a higher percentage of participants in the control group of our study were urban residents.
Patients in rural areas may be at risk of delayed diagnosis and treatment of BC due to limited access to medical care (18). However, Deuker et al. did not find significant differences in access to treatment or BC stage distribution according to residency status in the United States (19). Contrary to our findings, a study conducted by Toutounchi et al. on predisposing factors for BC in the population of Isfahan Province showed urban residence as a contributing factor to BC incidence (20).
Differences in study outcomes across various research can be attributed to factors such as diverse participant groups, geographical variances, sample sizes, and distinct regional risk factors. Methodological variations, including study designs and statistical approaches, can further influence results.
It should be noted that many people in rural areas use untreated or inadequately treated drinking water (well water), which contains more arsenic than urban purified water. Studies have shown that arsenic in drinking water increases the risk of bladder cancer (21, 22).
Agriculture plays an important role in the lives of the people of Guilan and is one of the most important economic sectors in northern Iran. This indicates a substantial agricultural presence and potential increased use of herbicides and pesticides for crop management in this area. This demographic distribution may result in differing environmental exposures and lifestyle habits between urban and rural residents, potentially affecting BC risk.
According to a study in 2022, Guilan Province is the oldest province in Iran, with 8.9% of the population aged 65 and over (23). Conditions like osteoarthritis, prevalent among the elderly, could intersect with solid cancers (24). Elderly individuals in rural areas, particularly those with rheumatic issues, tend to self-medicate, including opium use. The misapplication of pain-relieving substances, particularly among the elderly, might contribute to variations in BC incidence between Guilan's rural and urban zones.
In our study, a statistically significant association between smoking and BC incidence was observed in both healthy individuals and those diagnosed with BC. However, it should be noted that in the Iranian population, the number of female smokers in the studied age group is lower than that of males. A study on the relationship between smoking and BC incidence in Norwegian men conducted by Hadkhale et al. in 2018 corroborates the findings of our study (25).
Furthermore, our study reveals a statistically significant difference in the number of years of smoking and the number of cigarettes smoked per day between healthy individuals and BC patients, aligning with previous findings regarding the role of smoking in increasing cancer risk. This association remained significant after adjusting for other influencing factors, while the effects of other factors were not confirmed. The results of our study are consistent with the findings reported by Hadji et al. (26). Both studies demonstrated a statistically significant association between opium consumption and the number of years of opium use in both healthy individuals and BC patients. It should be noted that many opium consumers are also cigarette smokers.
Our results are consistent with the findings of Koushki et al. on the geographical distribution and risk factors for BC in Guilan Province (27). They reported that the risk of developing BC in Guilan Province was higher than the national average, with a 5-year incidence in Guilan exceeding the national average. Additionally, their findings established direct associations between smoking and BC risk in Guilan Province.
In our study, similar to many other previous reports (28, 29), a statistically significant association between different occupations and BC incidence was found (P < 0.001). Farmers had the highest risk of BC, followed by housewives and office clerks. It is evident that many factors, such as age, smoking, and opium consumption, within occupational groups can affect this relationship. Additionally, a statistically significant difference was observed in the number of years of employment among BC patients (P < 0.001). A nationwide case-control study in Iran reported a decreased risk for BC in male administrative and managerial workers and clerks (30).
A cohort study in Yazd, an industrial province in Central Iran, demonstrated a higher risk of BC in high-risk occupations such as metalworking, textiles, driving, farming, and construction (31). Similarly, another study in Iran showed an increased risk of BC among truck and bus drivers, skilled agricultural, forestry, and fishery workers, metal industry workers, domestic housekeepers, and construction workers (27). Additionally, Zaitsu et al. reported that occupation is a crucial independent determinant of BC survival in Japan (32).
In the present study, we identified a statistically significant association between occupations involving exposure to chemicals and BC incidence in both healthy individuals and those diagnosed with BC (P < 0.001). Our study revealed a significant association between exposure to combustion and greenhouse gases and a 10.72-fold increased risk of BC (P = 0.0001). Consistent with our findings, Yu et al. (33) highlighted the complex connections between climate change and cancer risks via modifiable risk factors. Climate change, with its influence on abnormal temperature, air pollution, and other factors, can exacerbate cancer inequities. Combustion processes and the associated emissions of greenhouse gases, such as carbon dioxide, are prevalent in various industries and activities, including energy production, transportation, and manufacturing.
Our study also found a significant association between exposure to tar and carbon derivatives and a 3.53-fold increased risk of BC (P = 0.0001). The IARC Working Group (34) reported evidence for the carcinogenicity of occupational exposures to bitumen and bitumen emissions, particularly in roofing and mastic-asphalt work, in humans. These substances, including tar and carbon derivatives, are commonly used in road construction, roofing, pavement-related occupations, as well as in industries involved in asphalt and carbon production.
Additionally, herbicides/pesticides, ammonium nitrate/explosives, aromatic hydrocarbons, and chlorophenylamine have all been shown to be significantly associated with an increased risk of developing BC (P = 0.0001). Herbicides and pesticides are frequently utilized in agriculture for crop protection. Ammonium nitrate and explosives are commonly employed in the mining and demolition industries. Aromatic hydrocarbons are prevalent in the petrochemical and chemical manufacturing sectors. Chlorophenylamine is used in the synthesis of certain chemicals and pharmaceuticals.
Furthermore, substances such as arsenic (P = 0.001), cresol (P = 0.002), smoke (P = 0.005), aluminum (P = 0.007), lead (P = 0.018), tire production materials (P = 0.022), methylene bis/chloroaniline (P = 0.033), and mustard gas (P = 0.043) have all demonstrated significant associations with BC. These chemicals are commonly encountered in industries such as mining, metalworking, chemical manufacturing, combustion-related workplaces, construction, rubber production, plastics, and, historically, chemical warfare.
Our findings revealed that while several occupations appeared to have elevated risks, the statistical significance of these associations varied. Notably, the p-values for specific chemical exposures, such as cutting fluids (P = 0.766), aromatic amines (P = 0.876), silica (P = 0.521), and asbestos (P = 0.39), did not meet the conventional threshold for statistical significance in our research.
Previous studies reported different results. For instance, a study by Hosseini et al. (30) observed an elevated risk of BC in male workers in occupations with likely exposure to aromatic amines and metal processors. Pourabdian et al. (35) showed a significant association between BC risk and occupations such as truck and bus driving, farming, metal industry work, domestic work, and construction.
Mazdak et al. (36) highlighted the role of chromium exposure as an oxidant element with a short half-life in BC development. Both mentioned studies were conducted in Isfahan, an industrial city in central Iran. Furthermore, the results of a case-control study by Latifovic et al. (37) in Canada, which investigated BC risk in men exposed to silica and asbestos, indicate a slight increase in the risk of BC with exposure to these agents, which did not support our study's results.
These discrepancies underscore the need for continued research to clarify the relationship between chemical exposures and BC risk. It should be noted that previous studies in Iran were performed in industrial cities with different climate and environmental conditions. Guilan Province, with its humid subtropical climate, lies along the sea, and the primary occupation of its people is agriculture. Consequently, the exposure to dangerous gases and occupational pollutants in this province is less than in the cities of central Iran. Therefore, the relationship between many pollutants and the risk of bladder cancer was not significant due to the lack of samples in these groups.
This study has some limitations. First, the most important limitation is the lack of control for intervening variables such as age and gender. Second, the small sample size did not allow for comparisons between men and women in different occupations or based on tobacco use.
Generally, a comprehensive cancer control program that unites governmental bodies, healthcare systems, insurers, academia, employers, and communities can foster reduced cancer incidence, enhanced patient quality of life, and public health advancement. For effective risk mitigation, targeted screenings and timely interventions are vital. Promoting education on occupational health and preventive strategies is essential for both workers and employers. Future research should encompass larger samples, meticulous variable control, and systematic reviews to fortify these findings.
5.1. Conclusions
Our findings demonstrate an increased risk of bladder cancer with exposure to greenhouse gases, tar, and carbon derivatives. Similarly, smoking, opium use, and well water consumption play a significant role in increasing the risk of bladder cancer.