1. Background
Pancreatitis, or inflammation of the pancreas, is a pathological condition caused by the infiltration of exocrine pancreatic enzymes, such as trypsin, phospholipase A, and elastase, into the pancreas (1). The pathophysiology of acute pancreatitis involves disturbances in both the extracellular and intracellular components of the pancreas due to obstruction in the transmission of pancreatic secretions, leading to the activation of pancreatic enzymes (2). Acute pancreatitis, characterized as an acute inflammatory process of the pancreas, is one of the most common gastrointestinal diseases that necessitate hospitalization and is generally associated with high mortality, particularly among the elderly (3).
Causes of acute pancreatitis include abdominal trauma, certain medications, viral infections, tumors, genetic abnormalities of the pancreas, elevated blood lipid profiles, gallstones, and alcohol consumption. Typically, chronic pancreatitis is associated with younger age groups and is less common after acute pancreatitis. The growing body of literature highlights the importance of identifying and treating the underlying causes of acute pancreatitis (4, 5). This study evaluates some comorbidities recorded in medical records of patients with acute pancreatitis. Acute pancreatitis is a leading cause of emergency general surgery requiring hospitalization. According to numerous studies, approximately 5% - 30% of cases of acute pancreatitis are idiopathic. Given that this disease progresses rapidly, clinical manifestations can vary (6, 7).
In review articles, the prevalence of pancreatitis ranges from 4.9 to 73.4 per 100,000 people and is usually associated with a good prognosis in 15 to 20 percent of patients (8). However, most deaths due to pancreatitis have been reported in individuals with multiple vital organ dysfunction following systemic inflammatory response syndrome (SIRS) and massive cytokine release within the first week (9).
A cross-sectional, retrospective analytical study was conducted on 160 patients with biliary pancreatitis at medical centers in Tehran from 2017 to 2020. It was noted that being female, having larger gallstones, a higher CRP level, and a high score on the Ranson criteria (10) were all independent risk factors for the development of biliary tract pancreatitis (11). According to a 2020 review study conducted in Iran on pancreatic malignancies, the incidence and mortality rates of pancreatic cancer increased steadily with age. Men were found to be more susceptible to this cancer than women, and smoking, advancing age, and lifestyle changes have been identified as the most significant risk factors for pancreatic cancer in Iran (12). An almost similar study conducted in Ahvaz about ten years ago stated that the highest rate of pancreatitis occurred in individuals aged 30 to 39. However, gender and age did not have a significant impact on the length of hospitalization or Ranson score (13).
2. Objectives
This paper aims to highlight the common causes of pancreatitis and factors affecting hospitalization.
3. Methods
The present study is a retrospective observational analytic study. Data were gathered from Hospital Information Systems (HIS) with the K85 code in the International Statistical Classification of Diseases 10 (ICD10). This study was approved by the University Research Ethics Board (IR.AJUMS.REC.1398.788).
Demographic information, comorbidities, laboratory parameters, and paraclinical information such as ultrasound documents and reports were collected. Inclusion criteria encompassed all patients treated for pancreatitis from 2017 to 2020 whose information was registered in HIS. The following patients were excluded from the study: those under 18 years of age, patients with metastatic tumors, acquired immunodeficiency syndrome, uremia, end-stage liver cirrhosis, active tuberculosis, resistant heart failure, previous transplantation, immunosuppressive therapy, pregnancy, chronic pancreatitis, and pancreatic carcinoma. Data normalization was evaluated using the Kolmogorov-Smirnov test. Qualitative variables were arranged using percentages and frequencies, while the mean and standard deviation were specified for quantitative data. Statistical analyses were performed using chi-square (or Fisher exact test), t-test (or Mann-Whitney), and simple logistic regression. Multivariate analysis was conducted using multiple logistic regression. Significance levels were set at 0.05.
4. Results
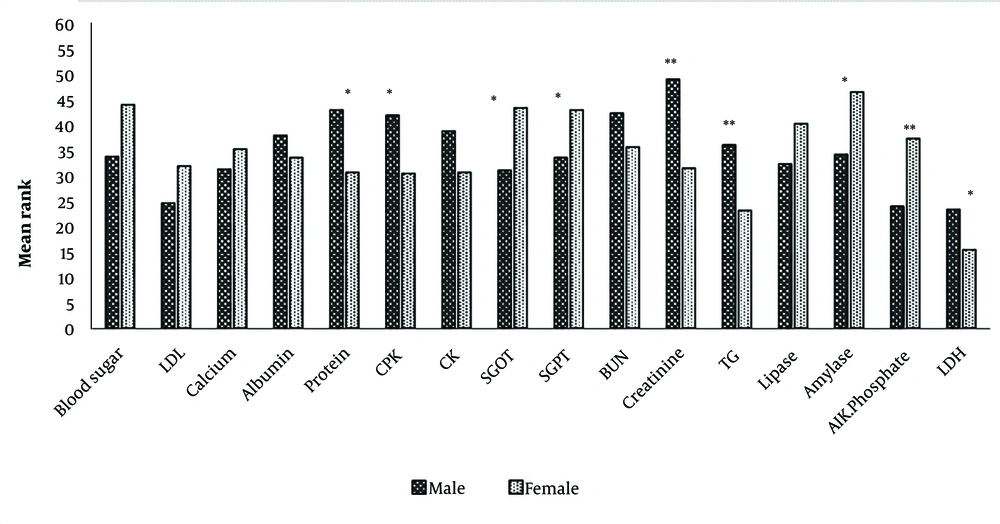
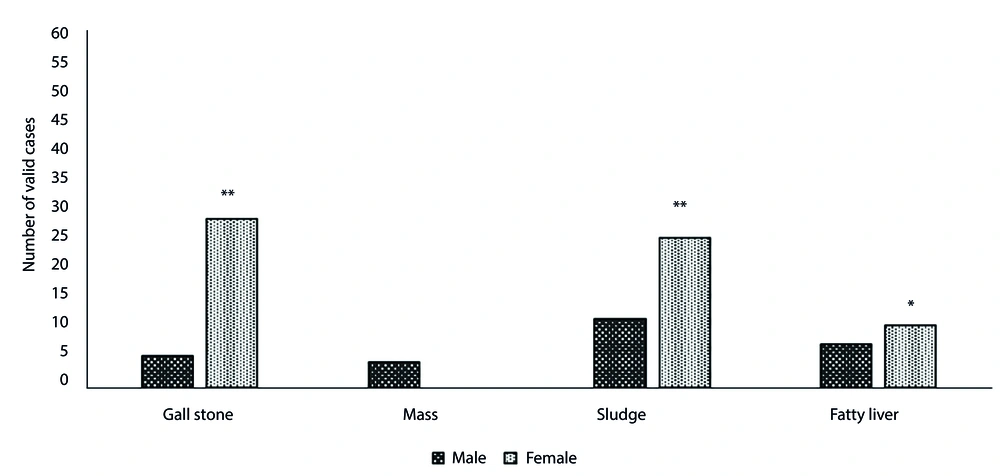
The initial analysis of patients diagnosed with pancreatitis over a 4-year period revealed that 84 out of nearly 99,467 patients were admitted during that time. The largest number of pancreatitis patients (36.9%) was hospitalized in 2020. Among them, the majority (53.6%) were aged 40 - 60 years, and women comprised a larger proportion of patients. In total, 85.7% of admitted patients were hospitalized for less than 10 days. About 92% were discharged with follow-up advice, while the remainder were discharged against medical advice (DAMA). No deaths were recorded during this period due to pancreatitis. Other specifications are shown in Table 1. Table 2 provides summary statistics for biochemical parameters. The correlation between hospitalization and quantitative variables was tested using the nonparametric correlation coefficient (Table 3). Pearson Chi-Square correlations were used to determine the relationship between hospitalization and nominal-qualitative variables. A significant positive correlation was found between blood glucose, creatinine, amylase, LDH, and hospitalization. As shown in Figure 1, differences between men and women were significant for protein, CPK, SGOT, SGPT, creatinine, triglycerides, amylase, alkaline phosphatase (ALP), and lactate dehydrogenase (LDH). The mean rank for ALP, amylase, SGOT, SGPT, and glucose was higher in women, whereas LDH, triglycerides, creatinine, CPK, and protein were higher in men. This study also compared men and women in terms of the presence of other comorbidities. Figure 2 illustrates a significant difference in the number of valid cases of gallstones, fatty liver, and sludge between men and women.
| Variables | No. (%) | Percent of Total Inpatients (%) |
|---|---|---|
| Date | ||
| 2017 | 12 (14.3) | 0.053 |
| 2018 | 17 (20.2) | 0.076 |
| 2019 | 24 (28.6) | 0.086 |
| 2020 | 31 (36.9) | 0.114 |
| Age (y) | ||
| 15 - 40 | 22 (26.2) | - |
| 40 - 60 | 45 (53.6) | - |
| ≥ 60 | 17 (20.2) | - |
| Gender | ||
| Female | 55 (65.5) | - |
| Male | 29 (34.5) | - |
| Hospitalization (Day) | ||
| ≤ 10 | 72 (85.7) | - |
| ≥ 10 | 12 (14.3) | - |
| Discharge with | ||
| Follow up | 77 (91.7) | - |
| Discharge against medical advice (DAMA) | 7 (8.3) | - |
| BMI | ||
| ≤ 30 | 47 (65.3) | - |
| ≥ 30 | 25 (34.7) | - |
| Gall stone | ||
| Yes | 52 (61.9) | - |
| No | 32 (38.1) | - |
| Troponin | ||
| Negative | 84 (100) | - |
| Positive | 0 (0) | - |
| Sludge | ||
| Yes | 49 (58.3) | - |
| No | 35 (41.7) | - |
| Mass | ||
| Yes | 4 (4.8) | - |
| No | 80 (95.2) | - |
| Fatty liver grade | ||
| No | 67 (79.8) | - |
| 1 | 10 (11.9) | - |
| 2 | 4 (4.8) | - |
| 3 | 3 (3.6) | - |
| Variables | Valid Data; (No.) | Min-Max | Mean ± SD |
|---|---|---|---|
| Blood Sugar | 84 | 77 - 500 | 157.79 ± 73.57 |
| LDL | 58 | 30.20 - 349 | 93.55 ± 53.18 |
| Calcium | 68 | 6.70 - 12.80 | 9.03 ± 0.85 |
| Albumin | 70 | 3.30 - 5.20 | 4.37 ± 0.40 |
| Protein | 70 | 4.50 - 10 | 7.12 ± 0.98 |
| CPK | 67 | 28 - 1851 | 148.49 ± 314.62 |
| CK | 65 | 7 - 55 | 19.83 ± 9.69 |
| SGOT | 78 | 15 - 847 | 189.52 ± 189.16 |
| SGPT | 78 | 8 - 843 | 210.97 ± 214.45 |
| BUN | 76 | 7 - 65 | 19.06 ± 11.34 |
| Creatinine | 76 | 0.50 - 6 | 1.15 ± 0.89 |
| TG | 55 | 42 - 647 | 127.58 ± 115.56 |
| Lipase | 74 | 30 - 4640 | 890.09 ± 900.67 |
| Amylase | 84 | 37 - 5856 | 1434 ± 1351 |
| Alk.Phosphate | 66 | 108 - 1587 | 434.66 ± 368 |
| LDH | 35 | 323 - 9000 | 872 ± 1434 |
| Nonparametric Correlations for Hospitalization | No. | Correlation Coefficient | Sig. (2-tailed) |
|---|---|---|---|
| LDL | 58 | -0.024 | 0.860 |
| Blood Glucose | 84 | 0.281 | 0.010 a |
| Calcium | 68 | -0.138 | 0.263 |
| Albumin | 70 | 0.053 | 0.661 |
| Protein | 70 | -0.111 | 0.361 |
| CPK | 67 | 0.108 | 0.383 |
| CK | 65 | 0.095 | 0.450 |
| SGOT | 78 | -0.046 | 0.691 |
| SGPT | 78 | -0.044 | 0.701 |
| BUN | 76 | 0.187 | 0.105 |
| Creatinine | 76 | 0.238 | 0.039 a |
| TG | 55 | -0.033 | 0.810 |
| Lipase | 74 | -0.010 | 0.934 |
| Amylase | 84 | 0.239 | 0.029 a |
| Alk.Phosphate | 66 | 0.141 | 0.257 |
| LDH | 35 | 0.548 | 0.001 a |
aP < 0.05 was considered statistically significant.
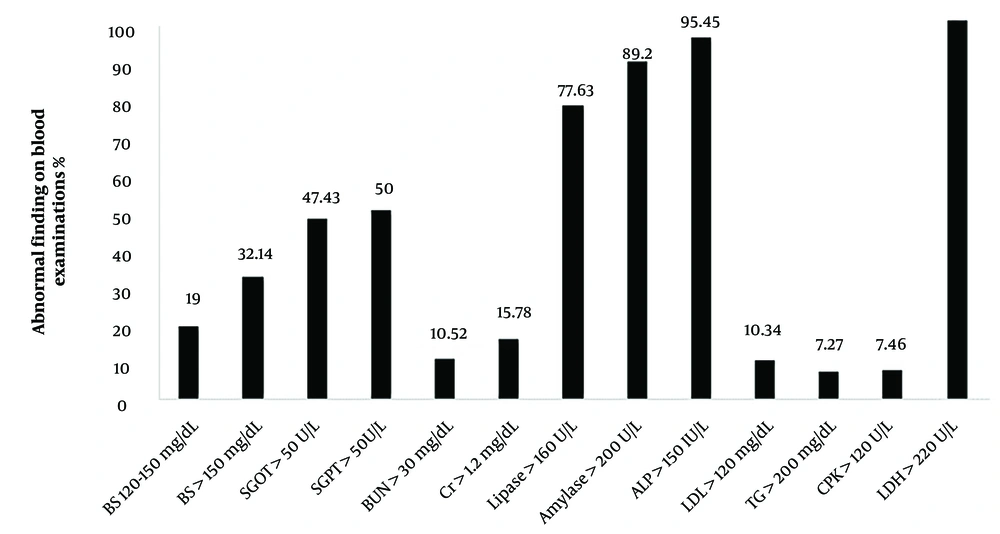
In the current investigation, some patients were discharged with a follow-up plan, while others chose to discontinue treatment at the medical institution and were discharged against medical advice (DAMA). Associations between discharge manner (Follow-up or DAMA) and quantitative variables were assessed using nonparametric correlation coefficients. Patients with high levels of CPK (P = 0.019) and creatinine (P = 0.03), and low levels of SGOT (P = 0.020) and SGPT (P = 0.030) were significantly more likely to be discharged with a follow-up plan. For qualitative variables, no significant relationship was observed between the parameters and discharge status. A pie chart illustrated the relative frequency of patients' comorbidities. Due to the limitations of table and image numbers, this chart is not mentioned in the manuscript. Abnormal findings from laboratory blood tests are compared in Figure 3. It is evident from this figure that more than 90% of patients had abnormal ALP, LDH, and amylase levels as expected. Conversely, less than 10% of patients had elevated levels of BUN, LDH, TG, and CPK.
5. Discussion
Although retrospective studies are less common, they can provide essential findings for planning prospective studies and designing clinical trials. A comprehensive meta-analysis conducted in 2020 by Iannuzzi et al. stated that the global incidence of acute pancreatitis has been continuously increasing over the past 56 years. The increase was 1.8% in the United States, 1.73% in Europe, and stable in Asia (14). Another systematic and comprehensive analysis focused on the global incidence and mortality of pancreatitis in 2021 by Ouyang et al. showed that in 2019, there were 2,814,972 cases of acute pancreatitis and 115,053 deaths from it. Additionally, the average age of infection has decreased by 5 - 10% from 1990 to 2019 (15).
Gallstones and autoimmune diseases are common symptoms in women diagnosed with acute pancreatitis, as evidenced by a review of other articles (5, 16, 17). The primary identified cause of acute pancreatitis is a blockage of the Oddi sphincter (18, 19). These findings confirm those from another study, suggesting that an obstructed pancreatic duct in laboratory conditions with animal samples can cause pancreatic obstruction (20).
Compared to men, women were more likely to have Sludge, which was present in 58.3% of patients in this study. There was no significant relationship between the presence of sludge and the duration of hospitalization. Studies on pancreatitis have shown that the risk of sludge and microlithiasis is not less significant than the presence of gallstones. It is important to conduct more detailed evaluations to treat the causes of this disease, prevent recurrence, and manage treatment when pancreatitis begins (21, 22). Although alcohol consumption is one of the most discussed and effective causes of pancreatitis, the patients' information about alcohol consumption in this study was incomplete and unreliable (23, 24). Hypertriglyceridemia was identified as the third most common cause of acute pancreatitis through a systematic review (25). A study conducted at a Chinese center with a large number of patients showed that it was the second most common cause (26). Another study found that every 100 mg/dL increase in serum triglyceride levels above 1000 mg/dl increases the probability of acute pancreatitis by 4% (27). A review study concluded that triglyceride levels at the time of admission, whether as the sole cause or as a comorbidity, conferred a worse prognosis; because the severity of acute pancreatitis associated with hypertriglyceridemia was worse than other causes (5). About 8% of the patients in the present study had TG levels above 200 mg/dl, and men were more likely to have higher levels than women. Lin et al. concluded that BUN determination after 24 hours of hospitalization has high accuracy for predicting the prognosis of acute pancreatitis, and BUN at admission has high accuracy for predicting in-hospital mortality (28). Talukdar et al. showed that an increase in BUN within 48 hours of admission can be a marker to predict the development of primary infection of pancreatic necrosis (29). Koutroumpakis et al. (30) enrolled 1612 patients with acute pancreatitis and found that elevated BUN in the first 24 hours of admission and HCT ≥ 44% were the most accurate predictors of persistent organ failure and pancreatic necrosis. However, in the current study, BUN was not significantly related to the length of hospitalization or survival. The increase in creatinine, amylase, and lactate dehydrogenase levels was statistically related to the duration of hospitalization.
Since 1997, advancements in diagnosis and treatment management have decreased the average length of hospitalization from 6.4 days to 4.7 days in 2017. Our study, similar to Crișu et al. (31), evaluates the correlation between hospitalization duration, an indicator of disease severity, and other variables. As mentioned before, the levels of blood glucose, lactate dehydrogenase, creatinine, and amylase had a direct and significant association with the length of hospitalization. Qualitative variables showed no significant association with the length of hospitalization. However, the age group of 40 to 60 years, female gender, presence of gallstones, and fatty liver were associated with an increase in hospitalization days. A study in 2022 reported similar results to ours, examining the relationship between lactate dehydrogenase levels and hospitalization duration (32). Also, similar results regarding the correlation between glucose levels and hospitalization period were observed in other articles (31-33). In this study, about 85% of the patients had a hospitalization period of more than ten days. Most articles indicate that there is a distinct group of patients who require longer-than-expected hospitalization. The demographic characteristics, comorbidities, primary cause, and laboratory variables of these patients are not significantly different from those of other patients. Instead, most of these patients experience prolonged hospitalization due to persistent abdominal pain and intolerance to oral refeeding (34).
5.1. Conclusions
The majority of comorbidities in this study were gallstones, sludge, and a BMI exceeding 30. Additionally, elevated levels of glucose, creatinine, amylase, and lactate dehydrogenase were associated with a prolonged period of hospitalization. These parameters can be used as tools to predict disease severity. During hospitalization, it is crucial to identify the exact cause of pancreatitis, as incorrect identification can lead to disease recurrence. Further research is needed to examine the predictive value of other biochemical parameters.
Some of the most significant limitations of this study include incomplete information for some patients in the HIS system, the small size of the investigated population, and a lack of trust in the recorded history of patients regarding lifestyle and alcohol consumption. Additionally, there is a paucity of studies and comprehensive articles in the geographical area under investigation.