1. Background
Valvular heart disease (VHD) poses a significant global public health concern, accounting for 2.5% of cardiovascular disease-related deaths (1). Rheumatic heart disease is consistently identified as the primary cause of VHD, affecting 41 million individuals worldwide, with low and middle-income countries experiencing the highest burden (2). Conversely, degenerative valvular diseases predominate in high-income countries (3), with calcific aortic and mitral valve (MV) diseases being the most commonly observed (4). The prevalence of VHD is on the rise due to various factors, including increased life expectancy and urbanization (5). The choice between medical and interventional therapies for VHD depends on several factors, with severe symptomatic valve dysfunction and asymptomatic patients with left ventricular systolic dysfunction often benefiting from interventional approaches, including surgical and transcatheter valve repair (6). While the use of transcatheter interventions for mitral (7) and aortic (8) valve repair has seen substantial growth in the last decade, surgical valve repair (SVR) remains the gold standard (9), and an essential tool for lifelong valvular disease management (8).
Paravalvular leakage is a well-recognized and common complication of SVR (10-12). To assess the valve repair quality and prevent such complications, cardiac surgeons employ transesophageal echocardiography (TEE) and pressurize the left ventricle using crystalloid fluid, also known as the saline test (13, 14). Although TEE is integral for intraoperative assessment (15, 16), it has two downsides. First, TEE must be performed with the patient off-pump, necessitating weaning from the cardiopulmonary bypass device (16, 17). Second, TEE must be performed by a trained and experienced specialist (16). In contrast, the saline test is a straightforward and widely accepted procedure, enabling visual inspection of the operation's quality and the identification of potential repair-related leaks (18, 19). However, the saline test's reliability diminishes in cases of aortic insufficiency, where left ventricular pressurization becomes challenging (17).
Given that trained and skilled echocardiologists might not always be available in the operating room, especially in areas with limited healthcare resources, cardiac surgeons occasionally rely solely on the saline test to assess the quality of the operation.
2. Objectives
In this study, we aimed to evaluate patients who underwent SVR without intraoperative TEE and gathered information about their cardiac and valvular function using transthoracic echocardiography (TTE).
3. Methods
The current study was conducted retrospectively, focusing on patients who had undergone prior SVR and were assessed using the saline test without intraoperative TEE before the commencement of our research. This study was performed at Imam Khomeini Hospital of Ahvaz between March 2019 and December 2022. The study protocol was approved by the ethics committee of Jundishapur University of Medical Sciences (IR.AJUMS.HGOLESTAN.REC.1402.035). Inclusion criteria encompassed all patients older than 18 years who underwent valvular repair surgery without intraoperative TEE, while patients younger than 18 years old or those who were reluctant or missed the follow-up session were excluded from the final analysis. The study protocol was thoroughly explained to all participants at the time of the study, and written consent was obtained. A cardiologist performed echocardiographic studies one week before surgery. Patients had undergone surgery with a midline sternotomy, and after valve repair completion, saline was injected through the MV using a bulb syringe to fill the left ventricle. A similar procedure was employed to assess tricuspid valve repair, with saline injected into the right ventricle. In cases of aortic valve repair, repair quality and cusp coaptation were evaluated by filling the aortic root with saline. Echocardiographic measurements were repeated during the follow-up session, which was planned for 12 months after valvular repair surgery. All surgeries and echocardiographic assessments were performed by the same team of surgeons and cardiologists.
3.1. Statistical Analysis
Descriptive statistics, including frequency and percentage for qualitative variables, and mean and standard deviation for quantitative variables, were used to summarize the data. Data normality was assessed with the Kolmogorov-Smirnov test. Paired samples t-tests or their non-parametric counterparts in cases of non-normally distributed data were performed using SPSS software version 27 (IBM Corp., Armonk, NY). P-values less than 0.05 were considered statistically significant.
4. Results
Initially, 18 patients were eligible for the final analysis. Two patients were excluded due to missing the follow-up session. Subsequently, a total of 16 patients, with a mean age of 63.8 ± 10.6 years and a male predominance (75%), were included in the study. Prior to surgery, 11 patients had normal sinus rhythm, while the remaining had an atrial fibrillation (AF) pattern on their ECG. One patient with AF prior to surgery experienced a rhythm change and achieved normal sinus rhythm postoperatively. The most common indication for valve repair was valve regurgitation/insufficiency (75%), and non-ischemia was the most common mechanism causing valve damage (56.2%) (Table 1).
| Variables | Values |
|---|---|
| Age (y) | 63.8 ± 10.6 |
| Gender | |
| Male | 12 (75) |
| Female | 4 (25) |
| History of diabetes mellitus | 7 (43.8) |
| History of hypertension | 7 (43.8) |
| History of dyslipidemia | 2 (12.5) |
| History of myocardial infarction | 3 (18.8) |
| Familial history of cardiac valve diseases | 2 (12.5) |
| Habit history | |
| Negative | 11 (68.8) |
| Smoke | 4 (25) |
| Alcohol | 1 (6.3) |
| Coronary angiography findings | |
| Patent coronary vessels | 4 (25) |
| 2-vessels disease | 3 (18.8) |
| 3-vessels disease | 9 (56.3) |
| Cause of valve repair | |
| Valve regurgitation/insufficiency | 12 (75) |
| Valve prolapse and flail | 2 (12.5) |
| Valve flail | 1 (6.3) |
| Valve prolapse | 1 (6.3) |
| Mechanism of valve damage | |
| Ischemic | 7 (43.8) |
| Degenerative | 5 (31.3) |
| Aortic dilation | 2 (12.5) |
| Rheumatism | 1 (6.3) |
| Functional | 1 (6.3) |
| Repaired valves | |
| MVr-TVr | 6 (37.5) |
| MVr | 5 (31.3) |
| AVr | 4 (25) |
| TVr | 1 (6.3) |
Abbreviations: MVr, mitral valve repair; TVr, tricuspid valve repair; AVr, aortic valve repair.
a Values are expressed as No. (%) or mean ± SD.
4.1. Comparison of Preoperative and Postoperative Valve Function Assessment
4.1.1. Mitral Valve Repair
Eleven patients underwent mitral valve repair (MVr). Eight patients had severe mitral regurgitation (MR), while the remaining three had moderate MR. With the exception of two cases, all other patients showed improvement in MV function (P-value: 0.006). Additionally, one patient with concomitant moderate mitral stenosis, associated with rheumatic heart disease, experienced complete resolution postoperatively (Table 2).
| Procedure and Dysfunction Severity | Pre-operation | Post-Operation | P-Value |
|---|---|---|---|
| Mitral valve repair (n = 11) | 0.006 | ||
| Normal | 0 | 1 | |
| Mild | 0 | 5 | |
| Moderate | 2 | 3 | |
| Severe | 9 | 2 | |
| Tricuspid valve repair (n = 7) | 0.01 | ||
| Normal | 0 | 1 | |
| Mild | 0 | 5 | |
| Moderate | 6 | 1 | |
| Severe | 1 | 0 | |
| Aortic valve repair (n = 4) | 0.10 | ||
| Normal | 0 | 1 | |
| Mild | 0 | 2 | |
| Moderate | 3 | 1 | |
| Severe | 1 | 0 |
4.1.2. Tricuspid Valve Repair
Tricuspid valve repair was performed on seven patients, six of whom also underwent MVr during the same surgery. One case had severe tricuspid regurgitation (TR), while the rest had moderate TR. All seven patients demonstrated improved tricuspid valve function (P-value: 0.01) (Table 2).
4.1.3. Aortic Valve Repair
Four patients underwent valve repair surgery due to aortic valve insufficiency (AI). Three cases had moderate AI, and one patient had severe AI. Three cases showed improvement in their aortic valve function, while one patient did not experience any improvement (P-value: 0.10). Additionally, one of the cases with normal mitral and tricuspid valve function preoperatively developed moderate MR and TR six months after the surgery (Table 2).
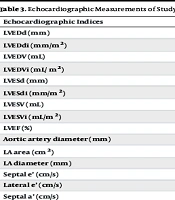
4.2. Echocardiographic Measurements
As presented in Table 3, by comparing echocardiographic measurements of study participants before and after surgery, significant changes were observed regarding left ventricular end-diastolic diameter (LVEDd) (P-value: 0.02), Left Ventricular End-Diastolic Diameter Index (LVEDdi) (P-value: 0.02), left ventricular end-diastolic volume (LVEDV) (P-value: 0.03), Left Ventricle End-Diastolic Volume Index (LVEDVi) (P-value: 0.02), interventricular septal diameter (IVSD) (P-value: 0.001), posterior wall thickness (PWT) (P-value: 0.02), tricuspid annular plane systolic excursion (TAPSE) (P-value: 0.002), right ventricular peak systolic myocardial velocity (RVSm) (P-value: 0.01), pulmonary artery pressure (PAP) (P-value: 0.002), interventricular septal thickness (P-value: 0.01), tricuspid regurgitation gradient (TRG) (P-value: 0.001), and right atrium (RA) area (P-value: 0.001). However, no statistically significant differences were observed regarding left ventricular end-systolic diameter (LVESd) (P-value: 0.68), left ventricular end-systolic diameter index (LVESdi) (P-value: 0.76), left ventricular end-systolic volume (LVESV) (P-value: 0.59), Left Ventricular End-Systolic Volume Index (LVESVi) (P-value: 0.73), left ventricular ejection fraction (LVEF) (P-value: 0.93), aortic artery diameter (P-value: 0.11), left atrial (LA) area (P-value: 0.10), LA diameter (P-value: 0.06), septal and lateral e’ (P-values: 0.52 and 0.40), septal and lateral a’ (P-values: 0.24 and 0.19), and right ventricular (RV) diameter (P-value: 0.11).
| Echocardiographic Indices | Pre-operation | Post-Operation | P-Value |
|---|---|---|---|
| LVEDd (mm) | 55.3 ± 8.4 | 51.4 ± 8.1 | 0.02 |
| LVEDdi (mm/m2) | 29.7 ± 4.2 | 27.7 ± 4.8 | 0.02 |
| LVEDV (mL) | 153.9 ± 6.3 | 130.4 ± 1.2 | 0.03 |
| LVEDVi (mL/ m2) | 82.1 ± 6.7 | 70.2 ± 6.4 | 0.02 |
| LVESd (mm) | 40.1 ± 9.9 | 40.4 ± 10.2 | 0.68 |
| LVESdi (mm/m2) | 21.5 ± 4.8 | 21.8 ± 5.8 | 0.76 |
| LVESV (mL) | 77.0 ± 10.2 | 78.3 ± 9.7 | 0.59 |
| LVESVi (mL/m2) | 42.1 ± 6.3 | 43.8 ± 7.0 | 0.73 |
| LVEF (%) | 42.1 ± 14.2 | 41.9 ± 14.5 | 0.93 |
| Aortic artery diameter (mm) | 34.6 ± 7.7 | 31.9 ± 4.0 | 0.11 |
| LA area (cm2) | 4.3 ± 0.5 | 4.1 ± 0.6 | 0.10 |
| LA diameter (mm) | 43.5 ± 5.4 | 41.6 ± 6.5 | 0.06 |
| Septal e’ (cm/s) | 7.2 ± 2.3 | 6.6 ± 1.5 | 0.52 |
| Lateral e’ (cm/s) | 8.8 ± 2.8 | 9.6 ± 1.7 | 0.40 |
| Septal a’ (cm/s) | 6.8 ± 3.1 | 6.0 ± 2.4 | 0.24 |
| Lateral a’ (cm/s) | 8.4 ± 3.5 | 6.8 ± 1.9 | 0.19 |
| IVSD (mm) | 10.9 ± 2.1 | 9.8 ± 1.7 | 0.01 |
| PWT (mm) | 9.9 ± 1.7 | 8.9 ± 1.4 | 0.02 |
| TAPSE (cm) | 18.7 ± 4.4 | 20.3 ± 4.5 | 0.002 |
| RV diameter (mm) | 33.2 ± 5.1 | 32.2 ± 4.6 | 0.11 |
| RVSm (cm/s) | 13.2 ± 2.6 | 8.2 ± 1.9 | 0.01 |
| PAP (mmHg) | 43.4 ± 12.1 | 32.9 ± 8.0 | 0.002 |
| TRG (mmHg) | 37.5 ± 11.1 | 27.5 ± 5.1 | 0.001 |
| RA area (cm2) | 18.8 ± 6.0 | 16.5 ± 4.8 | 0.001 |
Abbreviations: LVEDd, left ventricular end-diastolic diameter; LVEDdi, Left Ventricular End-Diastolic Diameter Index; LVEDV, left ventricular end-diastolic volume; LVEDVi, Left Ventricular End-diastolic Volume Index; LVESd, left ventricular end-systolic diameter; LVESDdi, Left Ventricular End-Systolic Diameter Index; LVESV, left ventricular end-systolic volume; LVESVi, Left Ventricular End-Systolic Volume Index; LVEF, left ventricular ejection fraction; IVSD, inter-ventricular septal diameter; PWT, posterior wall thickness; TAPSE, tricuspid annular plane systolic excursion; RV, right ventricle; RVSm, right ventricular peak systolic myocardial velocity; PAP, pulmonary artery pressure; TRG, tricuspid regurgitation gradient; RA, right atrium.
a Values are expressed as mean ± SD.
5. Discussion
Valvular heart diseases (VHDs) constitute a significant portion of cardiovascular diseases, responsible for 10 to 20% of cardiac surgeries in the United States (20). Valvular heart diseases can profoundly influence patients’ quality of life, physical ability, and mental well-being (2). In suitable candidates, surgical or percutaneous interventions can greatly improve their quality of life (21-24). Two common methods for intraoperative valve repair assessment are TEE and the saline injection test. Although TEE offers greater accuracy, the saline test is used when an experienced echocardiologist is unavailable, given its simplicity. Our study observed that patients who underwent mitral and/or tricuspid repair showed substantial improvements in valve function, while no statistically significant improvement was observed in aortic valve repair patients compared to their preoperative status. Furthermore, our patients experienced significant improvements in LVEDd, LVEDV, IVSD, PWT, TAPSE, RVSm, PAP, TRG, and RA area.
In our study, we observed substantial improvements in valve regurgitation severity among patients who underwent MVr and/or TVr. However, such notable improvements were not evident among patients who underwent aortic valve repair. Our findings align with previous studies that have explored the utility of the saline injection test in assessing valve repair quality. Chemtob et al. conducted a study involving 25 patients who underwent MVr using the saline test and reported a 100% success rate, with no or trivial MR observed on postoperative echocardiographic assessment (25). Similarly, Fujita et al. investigated 104 patients who underwent MVr using an irrigation device instead of the classic bulb syringe for the saline test and documented a significant reduction in MR severity postoperatively (26). Issa et al. observed that out of 20 patients who underwent MVr and had a satisfactory saline injection test, only one patient exhibited abnormal TEE results, necessitating additional procedures (27). Additionally, Abbasi et al. conducted a study involving 20 patients who underwent MVr and assessed repair quality using the saline test with occlusion of the left ventricular outflow tract. They reported that the findings of the saline injection test were consistent with TEE findings, demonstrating the feasibility and reliability of the saline injection test in this context (17).
In contrast to mitral and TVr, the use of the saline injection test in aortic valve repair is less common. This is due to the dynamic changes in aortic root pressure that occur under physiological conditions, with variations between systole and diastole. During the repair procedure, the heart is not beating, which poses challenges to the applicability of the saline test (28). Despite these challenges, our study observed improvements in aortic insufficiency severity in 3 out of 4 patients, with a decrease in the mean severity from 2.5 ± 0.5 preoperatively to 1.0 ± 0.81 postoperatively. However, this reduction was not statistically significant. Currently, the concept of devices designed to aid in aortic valve repair has been proposed, and ongoing research is exploring innovative approaches to address these complexities (28, 29).
The echocardiographic assessments of our study participants revealed noteworthy changes in various parameters, with reductions observed in LVEDD, LVEDV, LVEDDi, and LVEDVi following the postoperative period. However, LVESV, LVESVi, LVESD, and LVESDi did not exhibit significant changes. These findings align with the observations made by Gelfand et al., who reported changes in LVEDDi but not in LVESV among patients with chronic MR who underwent valve surgery (30). Similarly, Albini et al. reported similar findings in MR patients who underwent transcatheter MVr (31). Notably, three separate studies by Castleberry et al. (32), Trichon et al. (33), and Gangemi et al. (34), which focused on patients undergoing concomitant coronary artery bypass grafting (CABG) and MVr did not report significant changes in LVESV. Moreover, Cimino et al. did not observe alterations in LVESVi among patients who underwent transcatheter MVr (35).
In our study, no significant differences were found between preoperative and postoperative LVEF among study participants. While some studies have reported that valve repair procedures can improve LVEF (30, 31, 36, 37), there are also reports that failed to observe such changes. Nickenig et al. conducted a study on 30 patients who underwent TVr and found no significant improvement in LVEF (38). Kamperidis et al. similarly did not observe improvements in LVEF among patients who underwent MVr (39). Robiolio et al. reported that patients who had aortic valve repair did not exhibit any improvement in LVEF in the first week following their surgery (40). The observed discrepancies in left ventricular function indices between our study and those mentioned above may be attributed to differences in follow-up time. It is important to note that the left ventricle requires time to undergo remodeling, and echocardiographic indices are valuable measures of RV function widely utilized by clinicians. Numerous studies have highlighted the impact of successful MVr (31, 41-43) and TVr (42, 44, 45) on TAPSE measurements. Consistent with these studies, our participants displayed significant improvements in their TAPSE measurements. However, it is worth noting that TAPSE might underestimate RV function in patients who have undergone TVr (46). Recent advancements in echocardiographic techniques, such as three-dimensional speckle tracking echocardiography, offer the potential for a more precise estimation of RV function in these patients (47).
Common underlying causes of heart failure, increased PAP, and the development of pulmonary hypertension (PH) include MR, which stands out as a significant contributor to left heart failure. Repairing MR can lead to substantial improvements in cardiac function. Additionally, untreated TR can contribute to the worsening of PH. Successful MVr (31, 48) and TVr (49, 50) procedures have previously demonstrated their pivotal roles in controlling PH and reducing PAP. Furthermore, TRG has emerged as a valuable tool for screening PH (51). Ragnarsson et al. reported improvements in TRG among patients who had undergone MVr (43). Similarly, Sahebjam et al. showcased the positive effects of TVr on TRG by documenting a significant reduction in TRG among patients who underwent transcatheter tricuspid valve-in-valve replacement (52). In alignment with these studies, our research revealed significant improvements in both PAP and TRG measurements among our patients, consistent with studies that employed TEE for control and assessment.
5.1. Limitations
One of the limitations of the current study is the relatively small sample size. The limited number of study participants was due to the fact that most valvular repair surgeries were performed with the presence of an echocardiologist conducting TEE, and the number of surgeries performed without TEE was not high. We could not deprive patients of TEE to test our hypothesis due to ethical considerations. Therefore, we had to rely on the limited cases that underwent surgery without TEE. However, the research team is considering a follow-up session to collect data regarding the long-term effects of valvular repair without TEE and with the saline injection test. Additionally, the relatively small number of participants may affect the generalizability of our findings to a larger population. We did not assess cardiac function using more novel echocardiographic techniques, including 3D-STE, which could potentially highlight subtle changes in the ventricles. Moreover, the follow-up time of our study was relatively short, and studies with longer follow-up times are required to assess the quality and durability of valve repair outcomes over time.
5.2. Conclusions
In conclusion, the saline injection test has proven to be a valuable tool for cardiac surgeons when assessing the quality of mitral and tricuspid valve repairs, especially when TEE is not readily available. However, the reliability of this test in the context of aortic valve repair remains uncertain and warrants further investigation.