1. Background
Therapeutic plasma exchange (TPE) is a critical and life-saving treatment for diseases with a high load of pathogenic autoantibodies in circulation, including myasthenia gravis, chronic inflammatory demyelinating polyneuropathy (CIDP), multiple sclerosis (MS), and Guillain-Barré syndrome (GBS) in the field of neurology (1, 2). Plasma exchange is performed by separating and removing a significant volume of plasma from circulating blood and substituting it with fresh frozen plasma (FFP), albumin, or crystalloid materials, based on the clinical setting (3-5). Despite its efficacy and significant role in managing critical conditions, TPE is associated with several complications (6). Shock, dyspnea, coagulation disturbances leading to bleeding, electrolyte imbalances — particularly hypokalemia — allergic reactions, vascular access issues, as well as changes in medications’ metabolism and their blood levels, are key complications encountered in patients undergoing TPE (7). The development of complications and their characteristics depend on multiple factors, including the general condition of the patients (age, underlying diseases, current diagnosis, etc.), the number of TPE sessions, the type and volume of replacement fluid, and the access route. Although complication rates vary in the literature, overall complications of TPE in neurologic disorders are reported to be around 8 - 10% (8). In the study by Bobati and Naik, it was shown that in TPE patients, some abnormalities such as lack of access to the vein, fever, chills, and allergic reactions were observed (9). In another study, Tombak et al. showed that there were complications including tachycardia, blood pressure changes, allergic reactions, anxiety, and other factors in patients undergoing TPE (10).
To date, many studies have been conducted regarding the prevalence and types of complications in patients undergoing TPE. However, side effects have been evaluated at different time points after TPE. Given that most complications occur in the short term and after TPE, identifying these complications is important for patient care. The occurrence of complications within 24 hours after TPE can affect the patient's state of consciousness and lead to irreparable damage. Therefore, considering that limited studies have evaluated the occurrence of complications on days 1 and 5 after TPE, we investigated this issue in our study.
2. Methods
The present prospective cross-sectional study was conducted from October 2020 to October 2021 at the Neurology Department of Shariati Hospital, Tehran, Iran. In this study, all patients undergoing plasma exchange for any neurological indication were enrolled.
2.1. Inclusion and Exclusion Criteria
Inclusion criteria included patients with neurological disorders who required TPE. Patients unwilling to participate in the study, those with a history of TPE due to non-neurological causes, and those with severe underlying conditions (e.g., malignancies, severe uncontrolled heart failure) were excluded from the study.
2.2. Procedure
The study was approved by the Ethics Committee of Tehran University of Medical Sciences (IR.TUMS.MEDICINE.REC.1399.790). Plasma exchange treatment was carried out using an intermittent-flow centrifuge (Hemonetics PCS2) cell separator. A checklist form was completed for each patient, which included questions on age, gender, TPE indication (e.g., myasthenia gravis, GBS, CIDP), number of TPE treatment sessions, replaced plasma volume, vascular access site (peripheral vein, femoral vein, or jugular vein), consumption of anticoagulants before TPE (e.g., aspirin, clopidogrel), receiving anticoagulants during admission (e.g., heparin, enoxaparin), and receiving immunosuppressant agents (e.g., long-term prednisolone, azathioprine, cyclosporine). Complications were then checked, including cardiac complications, allergic reactions, infectious complications (catheter infection, sepsis), citrate-related reactions and hypocalcemia (paresthesia, cramp, tetany, and seizure), vascular injury (bruising, hematoma, pain, edema, and bleeding), technical complications (bleeding, pneumothorax, and thrombosis), coagulopathies (deep venous thrombosis, pulmonary thromboembolism), and anxiety. Patients’ outcomes were also collected. Complications were assessed on days 1 and 5 after undergoing TPE. Data related to complications in TPE patients were collected based on the information in their files. In addition, for each of the complications, such as cardiac complications or other cases, documentation related to clinical symptoms, tests, and examination results were evaluated by the relevant specialist. The data were collected from the patients’ records using a checklist that was prepared in advance. The data sampling method was available. Based on this, the files of patients whose data were complete were included in the study.
2.3. Statistical Analysis and Sample Size
Statistical analysis was performed using IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, NY, USA). Descriptive analysis is presented in the form of frequency and percentage or mean and standard deviation. An independent t-test and chi-square test were used for the comparison of continuous and categorical parameters at different time points. A P-value ≤ 0.05 was considered the threshold for statistical significance. To calculate the sample size, the following formula was used, which was chosen based on similar studies: P = 2.8%, d = 0.05, Z = 1.96. In the initial study, the number of patients was approximately 110; after the withdrawal of some patients, 95 patients were included in the study:
3. Results
3.1. Demographics and Baseline Characteristics
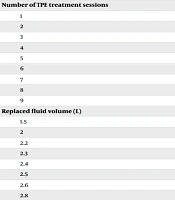
Ninety-five patients were evaluated in the current study. Forty-seven patients (49.5%) were female, while 48 patients (50.5%) were male. The most common indications for TPE were myasthenia gravis in 33 patients (34.7%), GBS in 27 patients (28.4%), and MS in 21 patients (22.1%). The majority of patients underwent 5 sessions of TPE treatments (60 patients, 63.2%), followed by 4 sessions in 15 patients (15.8%). The volume of replaced plasma was 2.5 liters in 53 patients (55.8%), with the next most common volume being 3 liters in 16 patients (16.8%). Vascular access was through a peripheral vein in 52 patients (54.7%) and a central venous line in 43 patients (45.3%). The replaced fluid was albumin in all patients. A history of previous anticoagulant therapy was present in one patient (1.1%), and immunosuppressant therapy was present in 39 patients (41.1%). The details of demographic and baseline characteristics are presented in Table 1.
| Parameters | No. (%) |
|---|---|
| Gender | |
| Female | 47 (49.5) |
| Male | 48 (50.5) |
| TPE indication | |
| Myasthenia gravis | 33 (34.7) |
| GBS | 27 (28.4) |
| MS | 21 (22.1) |
| Chronic inflammatory demyelinating polyradiculoneuropathy | 9 (9.5) |
| Myelitis | 4 (4.2) |
| Poliomyelitis | 1 (1.1) |
| Number of TPE treatment sessions | |
| 1 | 1 (1.1) |
| 2 | 2 (2.1) |
| 3 | 5 (5.3) |
| 4 | 15 (15.8) |
| 5 | 60 (63.2) |
| 6 | 7 (7.4) |
| 7 | 3 (3.2) |
| 8 | 1 (1.1) |
| 9 | 1 (1.1) |
| Replaced fluid volume (L) | |
| 1.5 | 1 (1.1) |
| 2 | 7 (7.4) |
| 2.2 | 1 (1.1) |
| 2.3 | 1 (1.1) |
| 2.4 | 1 (1.1) |
| 2.5 | 53 (55.8) |
| 2.6 | 4 (4.2) |
| 2.8 | 2 (2.1) |
| 3 | 16 (16.8) |
| 3.2 | 3 (3.2) |
| 3.3 | 1 (1.1) |
| 3.5 | 4 (4.2) |
| 3.6 | 1 (1.1) |
| Vascular access site | |
| CV line | 43 (45.3) |
| Peripheral vein | 52 (54.7) |
| Replaced fluid type | |
| Albumin | 95 (100) |
| Medication history | |
| Anticoagulant | 1 (1.1) |
| Immunosuppressant | 39 (41.1) |
Abbreviations: TPE, therapeutic plasma exchange; MS, multiple sclerosis; GBS, Guillain-Barré syndrome.
3.2. Results of Complications in Patients
Table 2 shows the complications that occurred in patients on the 1st and 5th day after TPE. On day 1, 10 patients (10.5%) experienced anxiety (P = 0.002). Additionally, 37 patients (38.9%) had citrate-induced hypocalcemia on day 1 after TPE (P = 0.012). By day 5, 31 patients (32.6%) exhibited albumin depletion (P < 0.001). This was despite the fact that no significant relationship was observed in terms of other side effects including albumin hypersensitivity, homeostasis, vascular access, pain and cardiac complications between days 1 and 5 (P > 0.05).
| Complications | Day 1 | Day 5 | P-Value |
|---|---|---|---|
| Albumin hypersensitivity | 1 (1.1) | 0 (0) | 0.915 |
| Citrate-induced hypocalcemia | 37 (38.9) | 0 (0) | 0.012 |
| Homeostasis | 14 (14.7) | 20 (21) | 0.125 |
| Orthostatic hypotension | 11 (11.6) | 16 (16.8) | |
| Nausea and vomiting | 3 (3.2) | 1 (1.1) | |
| Tachycardia | 0 (0) | 3 (3.2) | |
| Albumin depletion | 0 (0) | 31 (32.6) | < 0.001 |
| Hemostatic issues | 0 (0) | 0 (0) | - |
| Vascular access | 1 (1.1) | 0 (0) | 0.797 |
| Systemic infection | 0 (0) | 0 (0) | - |
| Pain | 7 (7.4) | 0 (0) | 0.119 |
| Cardiac complications | 1 (1.1) | 0 (0) | 0.813 |
| Anxiety | 10 (10.5) | 0 (0) | 0.002 |
a Values are expressed as No. (%).
4. Discussion
The TPE as a technique for the substitution of plasma antibodies and other immune factors with albumin, FFP, or other crystalloid materials can potentially lead to disturbances in coagulation, immunity, electrolyte balance, and drug levels, as well as hemodynamic instability and related technical issues such as catheter thrombosis, infection, or pneumothorax (11, 12). Thus, the safety of the procedure is a vital consideration in the management of the patient. In this study, we prospectively evaluated the complications of TPE in patients with neurologic disorders. The most common indications for TPE were myasthenia gravis (34.7%), GBS (28.4%), and MS (22.1%) in our study, which were more or less similar to other reports found in the literature. For instance, Kaya et al. (7) reported that 58% of patients undergoing TPE had GBS, 17.4% had acute diffuse encephalomyelitis, 7% had MS, and 6% had myasthenia gravis. Afzali et al. (13) also revealed that 34.1%, 33%, and 14.8% of patients treated with TPE had central demyelinating disorders, myasthenia gravis, and GBS, respectively. Thus, our findings regarding TPE indications are comparable to other studies in the literature. As in our study, the number of TPE treatment sessions was 5 (63.2%), followed by 4 (15.8%); other studies have also revealed similar figures. For instance, Baruah and Periyavan (14) reported that 68.9% of patients underwent 5 sessions of treatment. The volume of replaced plasma was 2.5 liters (55.8%) and 3 liters (16.8%) in the majority of our patients, but this amount was lesser in other reports. For instance, Baruah and Periyavan (14) reported 1.4 liters of fluid replacement in 5 sessions of TPE, and Gafoor et al. (15) revealed a volume of 1.23 liters for similar treatment sessions. The differences in this parameter between our findings and other studies can be attributed to various TPE protocols and study designs.
In the present study, different complications were observed in patients on days 1 and 5 after TPE. On day 1, some side effects, including albumin hypersensitivity, citrate-induced hypocalcemia, vascular access issues, anxiety, pain, and cardiac complications, were observed in the patients. The study by Vural et al. showed complications such as urticaria (29.1%), occlusion (3.2%), and faulty systems (1.01%) in patients during the first TPE (16). In the study by Nikkhah et al., the TPE process was performed on children with neurological disorders. The results showed that no complications were observed in the patients; however, the platelet count after TPE was reduced, which immediately returned to the normal range after a few days (17). In the study by Fodil et al., it was shown that coagulation disorders and infections were commonly observed in patients undergoing TPE. This was despite the fact that in the current study, the incidence of infection in patients was not reported as a complication (18). During TPE, a decrease in the level of plasma proteins is observed in patients. Therefore, it is necessary to increase the level of these proteins in the blood of patients by injecting blood products, including FFP. In the present study, one of the side effects observed in the patients was the decrease in albumin levels. Previous studies have shown that the reduction of plasma protein levels is one of the common side effects observed in patients undergoing TPE. This reduction can affect blood osmolality. By injecting blood products, especially FFP, the level of plasma proteins in patients can be increased (19, 20).
Anxiety and lack of access to the vein are other complications observed in patients undergoing TPE. In the present study, these two complications were observed in patients. In line with the present study, Barth et al. also showed that lack of access to the vein is usually observed in patients undergoing TPE. Those who undergo TPE for the first time experience more anxiety compared to those who have a history of TPE treatment (21, 22). The difference in the prevalence and type of complications in patients can be related to the conditions of the patients as well as their underlying diseases. Some patients undergo TPE due to infections or neurological disorders. Additionally, the age of patients can affect the occurrence of complications. Therefore, it is advisable for patients to use strategies to prevent complications based on their clinical conditions and the type of their disease.
4.1. Conclusions
In general, this study showed that complications such as albumin hypersensitivity, citrate-induced hypocalcemia, vascular access issues, cardiac complications, and anxiety occurred on day 1 after TPE in patients, while albumin depletion occurred on day 5 after TPE. This was despite the fact that homeostasis complications were observed in patients on both days 1 and 5 after TPE.
4.2. Limitations
This study has several limitations. First, the study is retrospective; it would be beneficial for future studies to be conducted in a prospective manner and to follow up with patients for a longer period. Additionally, a small number of patients were studied, and all patients were from a single center.