1. Background
Multiple sclerosis (MS) is a common neurological disorder affecting young adults, with onset typically occurring before the age of 16 or after 50. It is an inflammatory autoimmune disease with no definitive cure. However, disease-modifying medications, diet, and lifestyle adjustments can significantly improve quality of life (1). Studies estimate that MS affects approximately 2.3 million people worldwide, with a prevalence ranging from 50 to 300 per 100,000 individuals (2). Among Middle Eastern countries, Iran exhibits a notably high prevalence of MS (3). In most cases, MS initially presents as relapsing-remitting MS (RRMS), which may progress to a secondary progressive phase within 5 to 35 years (4-6). Given its chronic nature and associated symptoms, dietary interventions have garnered attention as potential adjunctive therapies.
The dietary approaches to stop hypertension (DASH) diet focuses on fruits, vegetables, whole grains, poultry, fish, and nuts while limiting saturated fats and sugary foods. This dietary approach may help reduce inflammation and improve blood pressure in individuals with MS (7). A cross-sectional study analyzing 37 million electronic health records revealed that hypertension is 25% more prevalent in MS patients than in those without MS, regardless of race (8).
The Glycemic Index (GI) measures the impact of food on blood glucose levels. Diets with a low Glycemic Load (GL), which emphasize fiber-rich, minimally processed carbohydrates, help stabilize blood sugar levels and may improve outcomes for individuals with MS. Benefits include maintaining lean mass during weight loss and reducing symptoms such as mood disturbances and fatigue (9).
Health messages play a critical role in promoting behavioral changes and increasing self-efficacy in individuals with MS (10). Message framing, which can be either gain-framed (emphasizing the benefits of a behavior) or loss-framed (highlighting the costs of not adopting it), influences behavior adoption. Positive messaging has been particularly effective in encouraging healthy habits, such as adherence to the Mediterranean diet, especially among individuals with low self-efficacy (11).
Positive framing focuses on the advantages of a recommendation, such as “Eating vegetables will improve your health”, whereas negative framing highlights the disadvantages, like “Not eating vegetables will harm your health”. Research demonstrates that positive framing is generally more effective in promoting healthy behaviors than negative framing (12). For example, positive messaging has proven successful in encouraging the consumption of fruits and vegetables (13).
Currently, no studies have specifically explored the use of persuasive and motivational messages to enhance knowledge and awareness among individuals with MS about the benefits of the DASH diet and low GI diets in alleviating stress, anxiety, and fatigue. This study aimed to examine the impact of gain- and loss-framed messages on promoting the DASH diet and low GI dietary practices among individuals with MS.
2. Objectives
2.1. Primary Goal
The primary objective is to evaluate and compare the average changes in fatigue levels within each of the three study groups and between the groups at baseline and at the conclusion of the study.
2.2. Secondary Goal
The secondary objective is to evaluate and compare the average changes in anthropometric data (weight and height), Body Mass Index (BMI), physical activity (PA), stress, anxiety, depression, and dietary intake within each of the three study groups and between the groups at baseline and at the conclusion of the study.
3. Methods and Results
3.1. Study Design
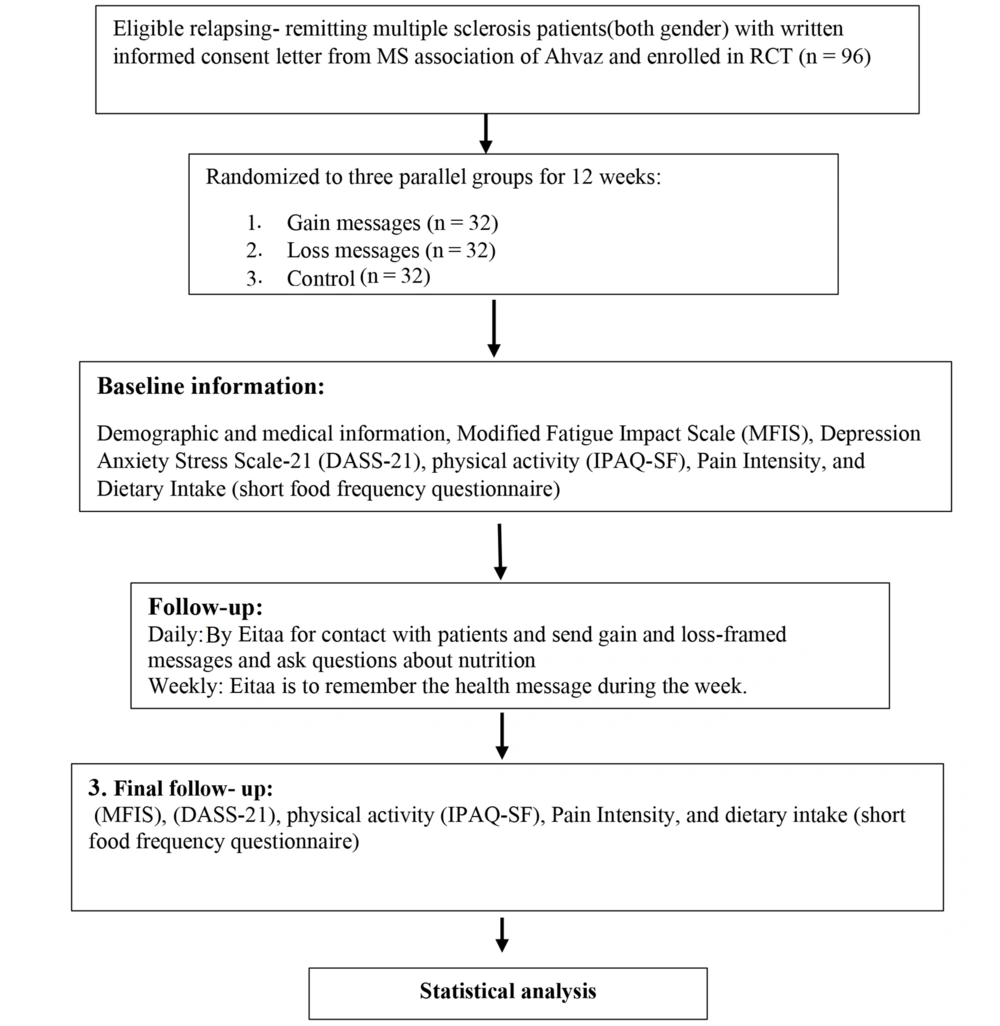
The present study was an open-label, 12-week, single-center, randomized controlled clinical trial involving patients with multiple sclerosis. The flow charts for the study were presented in Figures 1 and 2. The follow-up period lasted for 12 weeks.
This trial, dated June 17, 2023, was approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (ethical code: IR.AJUMS.REC.1402.165), and was registered with the IRCT code: IRCT20220902055853N3. The study was conducted at the MS Association of Ahvaz, Iran, and the results were disseminated through peer-reviewed publications and conferences.
3.2. Participants
Subjects were informed about the study through social media. Interested individuals were invited to the study center, where the investigator provided a detailed explanation of the study. After receiving clarifications, participants signed the consent form and underwent screening procedures for multiple sclerosis based on McDonald's criteria (14). Eligible subjects were then enrolled and randomized into study groups. The treatment duration was 12 weeks, with assessments conducted at baseline and at week 12 to evaluate the treatment effects.
3.3. Inclusion Criteria
- Both gender
- aged 18 to 55
- Willing to participate
- Stable in disease for 6 months
- No relapse in the past 30 days
- Internet access
- Own a smartphone
- Extended Disability Status Scale (EDSS) < 4.5
- Literate
3.4. Exclusion Criteria
- Have other types of MS or neurological diseases
- Concurrent participation in another study
- Illiterate or unwilling to read
- Use of insulin for diabetes control
- Menopausal
- History of kidney stones in the past 5 years
- Unwillingness to continue study participation
3.5. Sample Size
The sample size had been calculated based on a previous study (15) and the fatigue variable. The mean difference at the end of the study in the two intervention groups had been 12.39 ± 6.84, while in the control group, it had been 19.57 ± 5.20. Considering 99% power and a 95% confidence interval, a sample size of 32 participants per group had been determined. Minitab version 16 software had been used for the sample size calculation.
3.6. Randomization and Blinding
Initially, eligible participants were evaluated and randomized using permuted block randomization (with blocks of three) through the Random Allocation Software (RAS). The randomization list was generated and sorted based on random numbers. A total of 32 participants were assigned to the gain messages group, 32 to the loss messages group, and 32 to the control group. Due to the nature of the intervention, researcher blinding was not feasible, making this an open-label trial. To maintain the integrity of the process, the randomization list was generated by RAS and executed by an individual not involved in the study. Although the researchers were not blinded due to the educational focus of the study, the data analysts remained blinded to group assignments. Before participating, eligible participants were fully informed about the study's objectives and provided signed written informed consent.
3.7. Intervention Protocol
The experimental group received e-training interventions and robot-assisted exercises using digital technology to enhance efficiency and improve sensitivity. Online questionnaires were used for most assessments, except for the SH-FFQ, which was completed in person by a nutritionist. Patients provided a cell phone number for message delivery and follow-up via the Eitaa messenger app.
At the beginning of the study, patients completed various questionnaires covering demographic and medical information, fatigue, stress, anxiety, depression, physical activity levels, dietary habits, and pain intensity. Patients with MS were also asked about their preference for graphical or textual educational messages. Tailored messages were then designed to emphasize either the benefits or drawbacks of the DASH diet and low glycemic index on MS symptoms, according to their preferences.
3.8. Gain-Framed and Loss-Framed Messages
The gain-framed group received health messages emphasizing the benefits of the DASH diet and low glycemic index foods in managing MS symptoms. These messages highlighted how the DASH diet, which is rich in fruits, vegetables, whole grains, and lean proteins, could improve overall health and alleviate MS symptoms by enhancing energy levels and quality of life. Furthermore, the messages explained how low glycemic index foods, such as whole grains, legumes, and non-starchy vegetables, could help stabilize blood sugar levels, providing sustained energy to counter MS-related fatigue.
Conversely, the loss-framed group received messages focusing on the potential negative outcomes of not adhering to the DASH diet and low glycemic index recommendations. These messages stressed how neglecting these dietary guidelines could exacerbate MS symptoms, drawing on the latest nutritional guidelines and research (16-20).
3.9. Message Delivery and Assessment
A total of 64 health messages were delivered to both the gain-framed and loss-framed groups of MS patients over 12 weeks. Messages were sent on even days of the week, with two messages delivered on Saturdays and one message each on Mondays and Wednesdays for a duration of eight weeks. At the end of each week, the messages were reviewed cumulatively, and this process continued until the conclusion of the eighth week.
During the following four weeks, patients in both groups were asked to confirm whether they had read the messages. If any patient reported not having read the messages, all messages were resent individually. At the end of the 12th week, patients were asked to complete the Modified Fatigue Impact Scale (MFIS), Depression Anxiety Stress Scale (DASS), IPAQ, and SH-FFQ questionnaires again.
3.10. Discontinuation of Treatment
Researchers needed to discontinue a patient's participation in the project, noting the date and reason for withdrawal. If the patient agreed, the questionnaires scheduled for the 12-week endpoint were still completed. Participation was terminated if a patient chose to withdraw, relocated, or if adherence to the messages fell below 85% during the follow-up period.
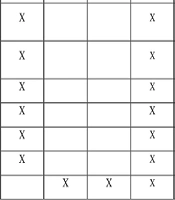
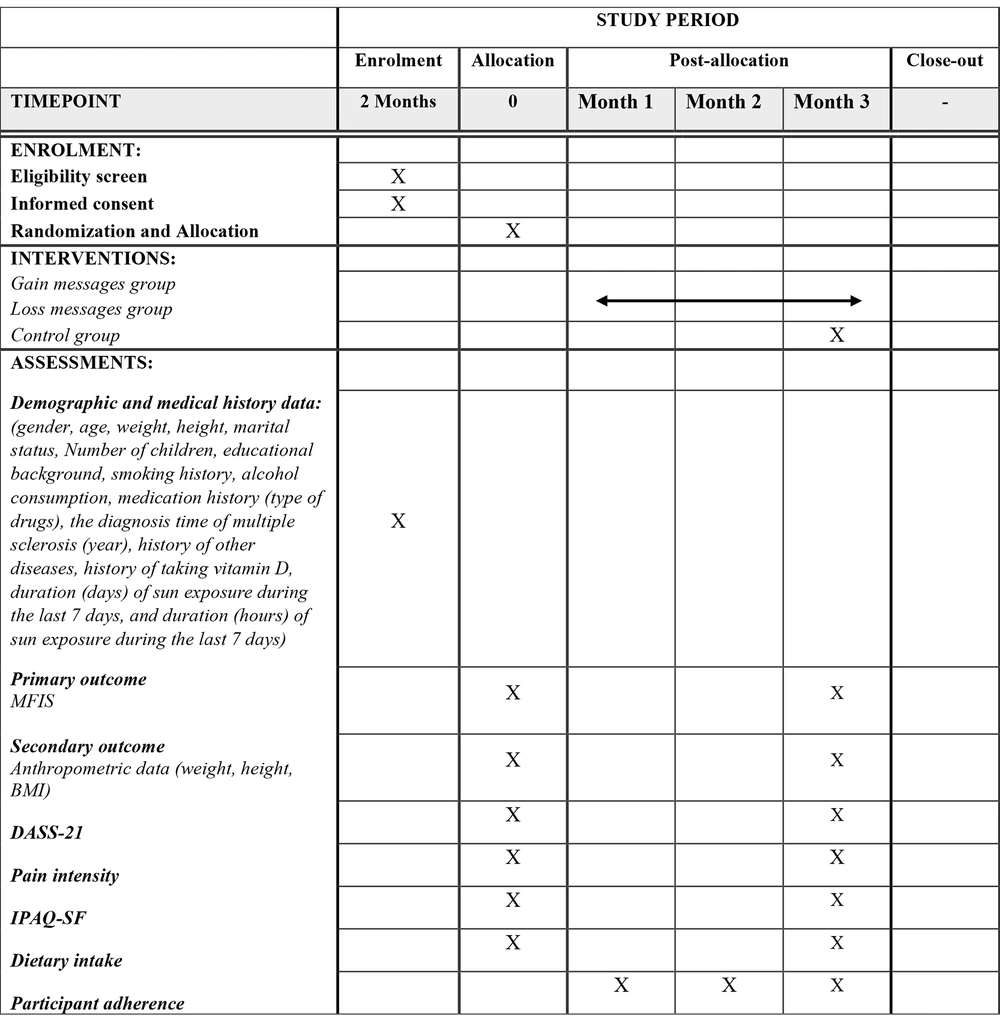
3.11. Outcome Measurements
All outcomes and time points are shown in Figure 2.
3.12. Data Collection
3.12.1. Demographic Background and Medical History
Data collected included gender, age, weight, height, contact information, marital status, educational background, smoking and alcohol history, medication details, year of MS diagnosis, history of other diseases, vitamin D intake, and recent duration of sun exposure.
3.12.2. Modified Fatigue Impact Scale
The MFIS assessed the impact of fatigue across physical, cognitive, and psychosocial dimensions, with a total score range of 0 to 84. Higher scores reflected a greater impact of fatigue. The study employed the validated Persian version of the MFIS (21).
3.12.3. Depression Anxiety Stress Scale-21
The DASS-21 evaluated depression, anxiety, and stress through 21 items divided into three subscales. Scores ranged from 0 to 21 for each subscale, with higher scores indicating greater psychological distress. This study utilized the validated Persian version of the DASS-21 (22).
3.12.4. Dietary Intake
Participants completed a validated 80-item Short Food Frequency Questionnaire (SH-FFQ) (23) to report their dietary intake over the past year. Nutritionists collected this data through face-to-face interviews at both the baseline and the end of the study. The Nutritionist IV software was used to estimate total calorie and macronutrient counts.
3.12.5. Physical Activity
The International Physical Activity Questionnaire (IPAQ-SF) was used to assess total weekly physical activity through face-to-face interviews. The following formula was applied to calculate physical activity (PA) in (MET minutes per week-1):
3.12.6. Pain Intensity
The Visual Analog Scale (VAS) was a validated tool for measuring acute and chronic pain (24). It involved marking a point on a 10-cm line that represented the continuum between "no pain" and "worst pain".
3.12.7. Glycemic Index and Glycemic Load
The glycemic index (GI) value of each food item was obtained from reliable sources, including the international tables of GI (16), the Iranian GI table (25), and the GI online database maintained by the University of Sydney (26). For food items lacking a documented GI value, estimates were derived based on their closest equivalents in terms of physical and chemical properties. White bread, assigned a GI of 100, served as the reference food for all derived GI values. Glycemic load incorporated both the quantity and quality of dietary carbohydrates (27).
GI and GL calculation the total dietary GI is calculated using the following formula:
Dietary GI = [(Carbohydrate content of food item) × (Number of servings/d) × (GI of food item)]/Total daily carbohydrate intake
Dietary GL = (Carbohydrate content of each food item) × (Number of servings/d) × (GI)
3.13. Monitoring
The study was supervised and monitored by a Project Manager (FB) and a Research Consultant (MA), who oversaw the study procedures to ensure data quality and adherence to the study protocol. The outcomes of the study monitoring were evaluated by a Data Monitoring Committee (DMC).
3.14. Patient and Public Involvement
Participants were invited and informed about the study's objectives and outcome measures. Daily reminders were sent to encourage them to read the messages, and nutrition counseling was provided if needed. The Data Monitoring Committee oversaw the study's progress and results, and participants received the completed outcome measurements as well as access to the trial publication.
3.15. Data Analysis
Data were reviewed for accuracy and completeness before proceeding with statistical analysis. Descriptive statistics summarized quantitative and qualitative variables, while paired t-tests, ANOVA tests, and ANCOVA tests were employed to compare group differences. The homogeneity of control and intervention groups concerning confounding variables was assessed. An intention-to-treat (ITT) analysis included all randomized participants regardless of dropout. SPSS version 26.0 (SPSS, Inc., Chicago, IL, USA) was utilized for both per-protocol sensitivity analysis and ITT analysis, with the significance level set at P < 0.05.
4. Discussion
The DASH dietary pattern is characterized by a high antioxidant capacity due to the consumption of food groups such as legumes, nuts, vegetables, and fruits, which can reduce inflammation and oxidative stress while improving brain function by influencing synaptic plasticity (28). Vegetables rich in folate and magnesium may contribute to enhanced brain responsiveness and exert anti-inflammatory effects (29, 30).
Higher dietary intakes of vitamin K, calcium, and potassium, along with lower sodium intake, as emphasized in the DASH diet, are independently associated with a reduced risk of depression, anxiety, and psychological stress through various mechanisms (31).
Glycemic load reflects both the quantity and quality of carbohydrates consumed (16). Additionally, this type of diet has been linked to a reduction in cardiovascular disease symptoms in individuals with MS (32).
A study demonstrated that a text message intervention significantly improved pregnant women's knowledge, attitudes, behavioral intentions, self-efficacy, practices, and dental plaque levels. The results indicated that text messaging positively influenced oral health outcomes compared to the control group, although message framing (gain or loss) did not affect the outcomes (33). Moreover, a systematic review of mobile text messaging interventions revealed that text messaging can significantly enhance health outcomes, self-management adherence, and health-related behavior change (34). Given the lack of sufficient studies and evidence among MS patients, evaluating the intervention effects of gain- and loss-framed messages combined with the DASH diet and a low- GI diet in MS patients is warranted.
4.1. Strengths and Limitations
The trial's strengths include the evaluation of gain- and loss-framed messages alongside DASH and low-GI diets in MS patients, utilizing digital technology for educational purposes. However, its limitations include reduced generalizability to other MS populations, as only RRMS subjects were selected, and the open-label design. Future research should address these limitations to encompass a more diverse MS population.