1. Background
In the last century, several pandemics were caused by different influenza virus subtypes, and there is a possibility that such epidemics will occur in the future. Coronaviruses were first described in humans in the 1960s (1). Since then, seven human pathogenic coronaviruses have been identified, including SARS-CoV, MERS-CoV, and the highly transmissible RNA virus SARS-CoV-2 (2). The SARS-CoV-2 virus originated in Wuhan, China, in 2019 (3). According to the World Health Organization dashboard, it has already caused more than 774 million infections and 7 012 986 deaths (4).
In the battle against this highly contagious disease, drugs and preventive approaches, including vaccination, were introduced. Vaccination against SARS-CoV-2 in individuals with multiple sclerosis (MS) was recommended to decrease the risk of complications from COVID-19 infection (5). It is assumed that vaccination can prevent infection or reduce disease severity, viral shedding, and thus transmission (6). Several vaccines against this virus have been developed and approved for public use in countries worldwide at an unprecedented pace (7). Some of the approaches used in developing a vaccine against COVID-19 were based on new technological platforms, employing mechanisms of action that differed from traditional methods (8).
However, the rapid development of vaccines has raised numerous questions about their efficacy and safety in various communities worldwide. A mixture of accurate and false information is circulating about COVID-19 and its vaccines, leading some individuals to have doubts (9). Additionally, incorrect online information has been disseminated during this period, deterring some people from getting vaccinated (10). Concerns regarding the safety, efficacy, and possible side effects (SE) of the vaccine have also arisen among healthcare workers. A regional review study, comprising 35 studies with a sample size of 76 471, revealed that this doubt among employees ranged from 4.3% to 72%, with an average of 22.51% (7). Nevertheless, studies have consistently shown that vaccination against COVID-19 is the most effective method to control the epidemic. Many countries, including the United States, have witnessed a decrease in cases and deaths as a result of widespread vaccination (11). Although vaccination has reduced deaths worldwide, examining its consequences in the general population, and especially in special populations, can provide valuable insights for future epidemics.
Multiple sclerosis is a chronic disease that affects many individuals. It is primarily caused by the immune system attacking the central nervous system (CNS). However, there appears to be a difference in the willingness and acceptance of the vaccine within this group, possibly due to the prolonged use of immunomodulation and immunosuppressive medications. A study conducted in Iran revealed that out of 892 participants, 68% expressed their willingness to receive the vaccine. However, the primary reasons for refusal were concerns over a potential reduction in the effectiveness of disease-modifying drugs (DMDs) and a general lack of trust in vaccines (12). Additionally, research has indicated that both SARS-CoV-2 infection and its corresponding vaccines could potentially uncover underlying CNS neuroinflammatory conditions (13). Findings from this study highlight the need to further investigate the consequences of vaccination through scientific evidence and to subsequently share this information with the scientific community.
Although there have been studies on the effects and consequences of vaccination on people with MS, most of these studies have been conducted in European and American countries. It is important to note that the type of vaccination, as well as the level of disease and the drugs administered, may differ in these regions. For instance, a case study on a 19-year-old man reported sudden death associated with potential relapses of MS following vaccination and COVID-19 infection (14). Another study, which included nine patients who had exhibited no signs of disease activity and had not altered their medications for a median of six years, showed new relapses of MS and neuromyelitis optica after receiving the AstraZeneca vaccination (15). In a longitudinal case-control study involving 65 participants, two groups were examined: 32 MS patients diagnosed after receiving the COVID-19 vaccine and a control group consisting of 33 vaccinated individuals without MS. The case group displayed demyelinating lesions in various areas (16). Additionally, a study of 559 vaccinated patients (476 complete, 74 partial, 9 indeterminate) revealed that 1.5% of patients experienced primary infection, and 11 individuals reported contracting COVID-19 after receiving partial vaccination (17). A retrospective, single-site cohort study of 250 MS patients concluded that the COVID-19 vaccine is safe for patients with MS, and cases of temporary worsening of MS symptoms following SARS-CoV-2 infection are rare (18). A systematic study of data from 4 417 patients concluded that there is no clinical evidence to support an increased risk of MS relapse or vaccination failure; however, the timing of vaccination should be adjusted on an individual basis (19). Another systematic review demonstrated that MS patients develop humoral and cellular immunity after infection with the COVID virus or vaccination, but the extent of the immune response depends on the specific disease-modifying therapies (20). Nonetheless, a review study has determined that further investigations are necessary to understand the role of cellular immunity in COVID-19 vaccination and the potential usefulness of booster doses (21). Additionally, another study notes that there is still insufficient long-term data on the effectiveness and safety of COVID-19 vaccines (8). Nevertheless, researchers are working to address the safety of vaccination in this population and have concluded that more experimental research is needed to determine the sustainability of reported safety (22).
2. Objectives
This retrospective cohort study was conducted, taking into consideration the specificity of the population—MS patients—and their receipt of different vaccines from various countries, with the goal of determining the disease course and recurrence of attacks following vaccination.
3. Methods
A retrospective cohort study was conducted on 302 patients with relapsing-remitting MS who had received the COVID-19 vaccine. These patients were referred to the MS clinic of two referral hospitals affiliated with Tehran University of Medical Sciences. The sample size was determined based on previous studies and a specific formula for estimating the prevalence of qualitative traits. The study aimed for a type 1 error level of 5%, a test power of 80%, and an accuracy of 0.015 in a population of 274 people. To account for possible dropouts, a total of 300 samples were collected.
After receiving approval from the Research Ethics Committee of Tehran University of Medical Sciences (IR.TUMS.MEDICINE.REC.1400.442) and obtaining permission from the hospital authorities to access the patients' files, we conducted face-to-face meetings with the patients to obtain their consent. Subsequently, a checklist was prepared and reviewed and approved by the research team regarding its content and the formulation of the questions.
Additionally, individuals who experienced major stressors such as divorce, the death of relatives, or any other significant stress at the time of vaccination, as reported by the subjects themselves, were excluded from the study. This was done to account for stress as a potential confounding factor in MS disease attacks. Furthermore, MS patients with cognitive impairment were not included in the study.
The checklist included demographic information and details related to the illness, such as the duration of the disease, underlying conditions, type and timing of vaccine injection, and the occurrence of attacks before and after vaccination. This checklist included questions about the symptoms of MS and when they occurred, specifically checking for symptoms from the previous month, three months ago, and six months ago. The checklist was completed during face-to-face interviews with the referred patients. The validity of the checklist was confirmed by five experts in the field.
Finally, the rates of relapse and attacks were compared one month, three months, and between three to six months after vaccination with the rates before vaccination. The data were entered into SPSS software. Descriptive and inferential statistics were utilized for data analysis. Descriptive statistics, including the frequency and percentage of attack recurrence over one, three, and six months, were examined. McNemar's tests were employed to identify differences between the two related groups in the binary dependent variable (attacked or not). Additionally, logistic regression was conducted to assess the impact of the binary dependent variables.
4. Findings
Among the 302 individuals examined, approximately a quarter (25.2%) were men. The ages of the participants ranged from 17 to 67, with an average age of 38.72 ± 9.05 years. The majority (88.7%) did not have any comorbidities; however, the most common comorbid condition was diabetes, which accounted for 3.6% of cases. This was followed by hypothyroidism (3%) and hypertension (2%). Out of the studied subjects, 172 individuals (57%) had a history of MS for more than five years, while the remaining participants had a history of less than five years. Approximately half of the samples (49%) reported pain at the injection site, while 54% did not experience fever after the injection. Additionally, around 36.8% of individuals experienced other symptoms such as hives, flu-like syndrome, and menstrual disorders after vaccination.
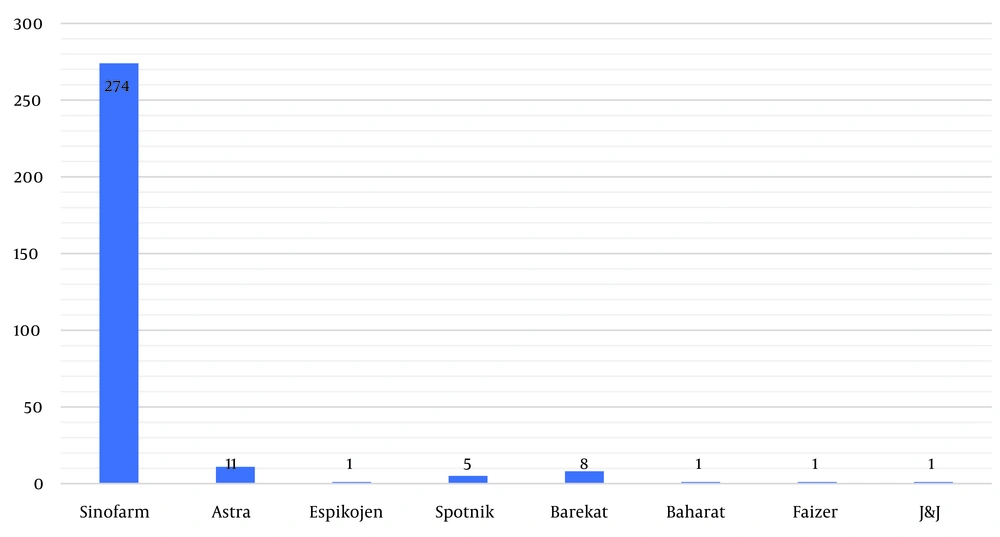
In the first round of vaccination, the majority (274 people, or 90.7%) received the Sinopharm vaccine. Oxford–AstraZeneca was administered to 11 people (3.6%), followed by the Iranian Barekat vaccine for 8 people (2.6%), and Sputnik for 5 people (1.7%) (Figure 1). In the second round, 8 people (2.26%) did not receive any vaccine, while the majority (263 people, or 87.1%) received the Sinopharm vaccine. The next most administered vaccine was Oxford–AstraZeneca, given to 14 people (4.6%), followed by Barekat for 7 people (2.3%), and Sputnik for 5 people (1.7%). Notably, in the third round, approximately one third (99 people, or 32.8%) did not receive any vaccine. Only 159 people (52.6%) received the Sinopharm vaccine. In the next category, 27 people (8.9%) received the Oxford–AstraZeneca vaccine, and 2% were vaccinated with Barekat. During the fourth round, the majority of individuals (259 people, or 85.8%) did not receive the vaccine. However, 8.3% (25 people) of those vaccinated opted for the Sinopharm vaccine. The next most common choice was the SpikoGen vaccine, selected by 2.6% (8 people), while only 1.7% (5 people) received the Pastocovacc vaccine. The remaining individuals received either the Oxford–AstraZeneca or Pfizer vaccines (Table 1).
| Type of Vaccine | First Injection | Second Injection | Third Injection | Fourth Injection | Total |
|---|---|---|---|---|---|
| Sinofarm | 274 (90.7) | 263 (87.1) | 159 (52.6) | 25 (8.3) | 721 (85.5) |
| Astra | 11 (3.6) | 14 (4.6) | 27 (8.9) | 4 (1.3) | 56 (6.7) |
| Espikojen | 1 (0.3) | 1 (0.3) | 5 (1.7) | 8 (2.6) | 15 (1.8) |
| Sputnik | 5 (1.7) | 5 (1.7) | 6 (2) | 0 (0) | 16 (1.9) |
| Barekat | 8 (2.6) | 7 (2.3) | 1 (0.3) | 0 (0) | 16 (1.9) |
| Baharat | 1 (0.3) | 1 (0.3) | 1 (0.3) | 0 (0) | 3 (0.3) |
| Faizer | 1 (0.3) | 3 (1) | 1 (0.3) | 1 (0.3) | 6 (0.7) |
| J & J | 1 (0.3) | 0 (0) | 0 (0) | 0 (0) | 1 (0.1) |
| Pastocovacc | 0 (0) | 0 (0) | 4 (1.3) | 5 (1.7) | 9 (1.1) |
| Total injection | 302 (100) | 294 (97.4) | 203 (67.2) | 43 (14.2) | 843 (100) |
| No Injection | 0 (0) | 8 (2.6) | 99 (32.8) | 259 (85.8) | 366 (100) |
| Total | 302 (100) | 302 (100) | 302 (100) | 302 (100) | 1209 |
a Values are expressed as No. (%).
It is important to note that, out of the total of 302 individuals, 34 (11.3%) were newly diagnosed with MS after receiving the vaccine. Among those who already had MS, 50% (151 people) were using Betacel-reducing drugs, while 38.7% (117 people) were taking other medications to manage their condition.
As shown in Table 2, 86.4% of participants had not experienced an attack in the six months prior to vaccination, while only 2.3% (7 people) had. None of the participants had an attack one month before vaccination. Four individuals experienced an attack within three months before vaccination, and three individuals had an attack between three and six months before vaccination.
| Attacks | First Month Before Vaccination | First Month After Vaccination | Three Months Before Vaccination | Three Months After Vaccination |
|---|---|---|---|---|
| No attack | 302 (100) | 173 (57.3) | 298 (98.7) | 151 (50.0) |
| Attack | 0 (0) | 95 (31.5) | 4 (1.3) | 117 (38.7) |
| New case | - | 34 (11.3) | - | 34 (11.3) |
| Total | 302 (100) | 302 (100) | 302 (100) | 302 (100) |
a Values are expressed as No. (%).
In 2020, only 6.6 million doses of the first vaccine were administered from September to March. In 2021, from March to June, 56.6% of individuals were vaccinated. The remaining individuals received the vaccine throughout the same year, with approximately one-fifth (23.9%) receiving it during the summer. By the end of the year, all individuals except for two had received the first dose of the vaccine.
For the second dose, only 5.3% had received an injection by March 2021. However, in the second quarter of the year, 42.7% had received injections. By the end of March 2022, 97.7% had received the second dose injection.
None of the samples received the third dose by March 2021. Additionally, 27.2% had not received this dose at all. In the spring of 2021, less than 1% received the third dose, and in the summer, 6% of the population received it. In the autumn, 23.5% received the third dose. However, except for 4 individuals, the majority had received this dose in the spring of 2022.
In most cases (70.9%), equivalent to 214 individuals, the interval between the first dose and the second injection was one month. Additionally, 18.3% of cases had an interval between two and three months, and a very small number of cases (3.6%) had an interval of five months or more between the first and second injections. Approximately 2% of people (6 individuals) had not received the second dose of the vaccine.
Out of the total sample, 135 individuals (44.7%) did not experience any attacks after receiving the vaccine doses. However, 41.7% of individuals (126 people) had an attack within three months after receiving one of the vaccine doses. Furthermore, 24 individuals (7.9%) experienced an attack between three and six months after vaccination. Only 5.6% of individuals had an attack after six months, which does not seem to be attributable to the vaccine. The number of attacks varied between 1 and 4 times for each person. Table 2 displays the number of attacks before and after vaccination, while Table 3 compares them. Table 4 shows the frequency of attacks according to the vaccine injection phase.
| Attack or New Case After Vaccination | Attack or New Case Before Vaccination | Total | ||
|---|---|---|---|---|
| Without Previous MS | Less Than 6 Months Ago | More Than 6 Months Ago | ||
| No attack | 0 (0) | 5 (71.4) | 130 (49.8) | 135 (44.7) |
| To 3 months after vaccination | 29 (85.3) | 2 (28.6) | 95 (36.4) | 126 (41.7) |
| Between 3 - 6 month | 4 (11.8) | 0 (0) | 20 (7.7) | 24 (7.9) |
| More than 6 month | 1 (2.9) | 0 (0) | 16 (6.1) | 17 (5.6) |
| Total | 34 (100) | 7 (100) | 261 (100) | 302 (100) |
Abbreviation: MS, multiple sclerosis.
a Values are expressed as No. (%).
| Variables | First Injection | Second Injection | Third Injection | Fourth Injection |
|---|---|---|---|---|
| No attack | 277 (91.7) | 246 (81.5) | 137 (45.4) | 31 (10.3) |
| Attack | 25 (8.3) | 48 (15.9) | 66 (21.8) | 12 (4) |
| Without injection | 0 (0) | 8 (2.6) | 99 (32.8) | 259 (87.7) |
| Total | 302 (100) | 302 (100) | 302 (100) | 302 (100) |
a Values are expressed as No. (%).
McNemar's statistical test showed significant results for the one month before and after vaccination. Additionally, the test was significant for the following three months.
The relapse rate for patients using the B-cell inhibitor drug one month after injection was 44%, compared to 24% for patients using non-B-cell inhibitor drugs (P < 0.001). After three months, the relapse rate was 50% for patients on the B cell inhibitor and 34% for those on the non-B-cell inhibitor drug (P = 0.005), which is statistically significant.
The findings showed that there is no significant relationship between the recurrence of attacks and factors such as the number of vaccine injections, age, gender, underlying disease, or the type of injected vaccine (P > 0.05).
The results also indicate that 76% (228 people) who received the Sinopharm vaccine for the first time continued to receive the same vaccine in subsequent rounds. Out of the 11 people who initially received the Oxford–AstraZeneca vaccine, 9 continued to receive the same vaccine in the following rounds. However, some individuals did not receive consistent vaccines in every round or at least in one of the rounds. Fisher's exact statistical test did not show a significant relationship between attacks and categories related to the type and order of vaccination (P = 0.30).
5. Discussion
The findings of this study revealed that the majority (74.8%) of the samples were women, with an average age of around 40 years. Furthermore, it was observed that most participants did not have any comorbidities. However, among those who did, diabetes was the most prevalent. Additionally, over half of the participants had a history of MS for more than 5 years. The aim of this study was to investigate complications following injection in the sampled population.
About half of the samples experienced pain at the injection site and fever, while more than a third suffered from other symptoms such as hives, flu-like syndrome, and menstrual disorders after vaccination. Although these studies were conducted on different vaccines, at different times, and differed from the current study, the results were generally consistent. The most common complications reported included arm pain (between 85% and 54%), flu-like symptoms, headache, fatigue, malaise, mild fever, body pain, as well as muscle or joint pain (5, 17-20). However, another study revealed that systemic symptoms such as fever, headache, and fatigue were more prevalent in individuals with MS compared to healthy individuals (59.3% vs. 21.1%) (21). On the other hand, a retrospective study on the AstraZeneca (AZD) and BNT162b2 vaccines demonstrated that while a significant number of individuals with MS experienced symptoms after receiving the first and second doses, these symptoms resolved quickly. There were no reports of severe SE, and post-vaccination symptoms were similar to those experienced by the general population, often being temporary (22).
The current study revealed that 86.4% of participants had not experienced an attack in the six months prior to vaccination. Only 1.3% (equivalent to four individuals) had an attack in the three months leading up to vaccination, and none of the participants had an attack one month before vaccination; they remained attack-free. However, within three months after receiving the injection, 38.75% (equivalent to 117 people) experienced an MS attack, and 31.5% (equivalent to 95 people) had an attack in the first month.
A retrospective observational cohort study conducted in Prague examined 1,661 vaccinated MS patients without a history of COVID-19, alongside 495 unvaccinated MS patients who had previously contracted COVID-19. The study found a slight increase in relapse incidence following COVID-19 vaccination, concluding that there is a slight increase in the incidence of MS relapse after COVID-19 vaccination. However, the increase in the incidence of MS relapse was higher after a COVID-19 infection. One difference between this study and the current study is that 90% of the patients in the Prague study received the BNT162b2 (Comirnaty) mRNA-type vaccine, while in the current study, most of the participants received the Sinopharm vaccine (23). In another study, 42% of MS participants vaccinated with mRNA vaccines reported SE after the first and second doses, respectively. A higher rate of SE was observed in the MS group after the second dose (67% vs. 45%). However, in the same study, when comparing vaccinated and unvaccinated MS/NID patients, there was no difference in rates of recurrent neurological symptoms, new neurological symptoms, or new/active lesions detected on MRI (17).
In the current study, 34 new cases of MS were diagnosed after receiving the injection. This may be attributed to the mild and unclear symptoms of MS, which could have resulted in underdiagnoses, or it could be linked to the vaccine itself. Further research is necessary in this area to reach a more conclusive understanding. While only a few studies have noted new cases of MS, a literature review uncovered 173 instances of neuroinflammatory CNS diseases, such as myelitis, optic neuritis, and encephalomyelitis, following SARS-CoV-2 infection or vaccination. The majority of these cases emerged within 2-3 weeks post-infection or vaccination. However, more than 70% of each phenotype in the literature were associated with infection (13). In our study, the highest incidence was observed within one month after vaccination.
Of the individuals who already had MS, 50% used Betacel-reducing drugs, while the remaining 38.7% used non-Betacel-reducing drugs. The recurrence of the disease in users of Betacel-reducing drugs one month and three months after injection was higher and statistically significant compared to users of non-Betacel-reducing drugs. In another study, 44 individuals with MS who were treated with alemtuzumab were investigated. The researchers concluded that there is no evidence of an increased risk for severe COVID-19 in individuals with MS who were treated with alemtuzumab (24). Additionally, a systematic review in this area explored how B-cell-reducing treatments may accelerate post-vaccination humoral waning (25).
A retrospective study involving 114 patients from an MS center confirmed that individuals who undergo anti-CD20 infusion therapies may experience a disadvantage in humoral immunity after receiving vaccinations (26). Furthermore, other researchers have emphasized that the increased risk of a severe course, including death, after SARS-CoV-2 infection in MS is not due to the disease itself, but rather to the use of anti-CD20 monoclonal antibodies in MS (26). However, another study found no significant correlation between the use of disease-modifying therapy (DMT) and the SE of the COVID-19 vaccine (5).
These multiple results indicate the need for further studies in this field. For certain DMTs, it may be beneficial to align the timing of the vaccine with the DMT dose to enhance vaccine effectiveness (27). One study noted a slight uptick in adverse events after vaccination in patients on immunosuppressive drugs. As a result, it has been suggested that the BNT162b2 COVID-19 vaccine is safe for individuals with MS (28). The researchers presented the results of a meta-analysis involving a total of 6 860 patients with multiple sclerosis (pwMS) to evaluate the impact of DMTs on the immune response to COVID-19 vaccines. Overall, pwMS receiving anti-CD20 and sphingosine-1-phosphate receptor modulator (S1PRM) treatments showed a decreased serologic response after full vaccination compared to those not receiving DMTs (29). The researchers have recommended serological surveillance after vaccination.
In this study, there was no difference in the incidence of complications between different vaccines. However, in another study conducted in the UK on the SE of the AstraZeneca (AZ) and Pfizer (PF) vaccines in individuals with multiple sclerosis (pwMS), 34.5% (388 out of 1,154) experienced complications with the PF vaccine, while 58.4% (822 out of 1,408) experienced complications with the AZ vaccine (21). Another study found that individuals who received the Moderna vaccine reported higher reactivity after the second injection compared to those who received the Pfizer-BioNTech vaccine (18).
The discrepancy in results between the current study and the aforementioned studies can be attributed to the differences in vaccines used. In the current study, the most commonly used vaccine was Sinopharm, while the Pfizer and Moderna vaccines were not utilized.
The findings of the current study revealed that there is no significant relationship between the recurrence of attacks and age, gender, underlying disease, or type of injected vaccine at any stage of vaccination (P > 0.05). However, a separate study indicated that a younger age was linked to MS recurrence following vaccination or infection (23). Additionally, another study found that being younger and female were associated with experiencing a reaction after the initial vaccine dose (18). Moreover, a study involving 193 individuals who received either the AZD1222 or BNT162b2 vaccine found that men had a lower risk of reporting symptoms after the first vaccination (22). On the other hand, a study involving 555 patients who received the BNT162b2 vaccine showed a slight increase in the rate of SE among younger patients (aged 18 to 55 years) (28). Nevertheless, another study noted that there is no evidence suggesting that individuals with MS are at a higher risk for complications from mRNA, non-replicating viral vector, inactivated virus, or protein COVID-19 vaccines compared to the general population (27).
5.1. Conclusions
The conclusion drawn from this study, along with other studies, is that despite the rapid expansion of vaccines and some stages lacking full approval, they are still highly recommended compared to the severe complications of the disease. The studies have shown that the risks associated with COVID-19 are greater than the potential risks of the vaccine (27). Therefore, it is advised that all individuals with MS follow the general recommendations of the World Health Organization and get vaccinated against SARS-CoV-2, based on regional recommendations and the advice of their treating neurologist. Any adjustments to their treatment should be made accordingly (30). The findings from this and similar research can be valuable for addressing epidemics and regional diseases. While the results obtained from this study can be linked to the vaccine, the study is retrospective. Therefore, future studies on the long-term effects of the vaccine are needed to generalize the results.
5.2. Limitations
Due to specific circumstances, this study was conducted as a retrospective cohort study. Each participant was compared with their period before receiving the vaccine. However, there was no opportunity to compare participants with individuals with MS who did not receive the vaccine. This limitation was taken into consideration when interpreting the results of this study.