1. Background
While multiple sclerosis (MS) is the most common cause of non-traumatic neurological disability in young adults (1), environmental factors are increasingly recognized for their role in disease onset, particularly in regions like Khuzestan. The prevalence of this disease is twice as high in women as in men, and the average age of onset is 30 years (2). It is assumed that, in addition to genetics, environmental factors may play a significant role in the etiology of the disease, as geographical location has a substantial impact on the prevalence of MS. The MS is considered uncommon in tropical and subtropical regions (1). According to statistics, Africa and Asia have the lowest prevalence rates, but recent studies suggest an increase in prevalence in these regions (3).
Although Iran has historically had a low prevalence of MS, it has recently become a country with a high prevalence, and the numbers continue to rise (4, 5). In 2013, while the global prevalence of MS was reported as 73 per 100,000, Iran reported a prevalence of 60 per 100,000 (6). In 2015, Dehghani et al. observed an increasing trend in all provinces of Iran over a five-year study period (7). Several review studies have reported MS prevalence rates in Iran ranging from 5.3 to 89 per 100,000 and incidence rates ranging from 7 to 148.1 per 100,000 (4, 6).
The exact cause of the increase in MS prevalence remains uncertain. The role of environmental factors in MS susceptibility has been explored in limited studies. Among these factors, the effect of air temperature on the disease is noteworthy. A study by Ebrahimi and Sedighi in various cities of Kerman province found a linear relationship between air temperature and MS prevalence, with higher prevalence reported in colder climates (8). Similarly, Spelman, studying 30 countries worldwide while considering geographical latitude, demonstrated a pronounced seasonal recurrence pattern with an annual sinusoidal trend. This pattern peaked in early spring and declined in autumn in the northern hemisphere, with extensions observed in the southern hemisphere (9).
Furthermore, a study analyzing monthly average meteorological data revealed significant seasonal variations in ambient temperature, relative humidity, and wind speed. The monthly onset distribution of MS in the study group was highest in May and June (12.2% each) and lowest in September (3.7%) (10). However, contrary findings were reported in Israel, where Saaroni et al. observed no significant effect of meteorological parameters, season, month, or specific days on MS relapse occurrence (11).
Air pollution is another critical environmental factor linked to MS. In Tehran, Heydarpour et al. demonstrated that long-term exposure to air pollutants, including PM10, SO2, NO, NO2, and NOx, may act as risk factors for MS (12). Supporting this, a similar study conducted in Italy in 2016 suggested that air pollution might contribute to MS onset and relapse. Increased exposure to PM10 was associated with higher hospitalization rates for the disease (13).
Although the exact cause of MS remains unknown, a combination of factors, including environmental influences, infections, genetic predispositions, immune system dysfunctions, viruses, toxins, metabolic issues, and substances like nitric oxide, may play a role in its development.
2. Objectives
The 2013 annual report by the World Health Organization identified Ahvaz as the most polluted city in the world, with an annual mean PM10 concentration of 372 μg/m³ (14-16). Due to industrialization, global warming, and Khuzestan province's exposure to dusty weather conditions, this study aimed to quantitatively assess the relationship between exposure to dusty days, temperature, and wind speed and the prevalence of MS, focusing on identifying critical thresholds. Epidemiological information can assist in the rapid identification, diagnosis, and control of the consequences of this disease (17).
3. Methods
Khuzestan province, located in southwest Iran along the Persian Gulf coast, is a key center for the country’s oil and gas extraction activities. Covering an area of 64,055 square kilometers and housing a population of over 4,711 million (based on the 2016 census), it is the fifth most populous province in Iran. Geographically, Khuzestan is situated at coordinates 31.33˚N and 48.69˚E (18).
This ecological study was conducted in Khuzestan province in 2019 using available data. Ecological studies are particularly suitable for evaluating population-level associations between environmental factors and disease prevalence.
The dataset included the prevalence of MS, the number of dusty days, the average daily temperature, and wind speed for the 27 counties of Khuzestan province, Iran, all collected in 2019.
Participants were identified using data from the Khuzestan MS Center, affiliated with Ahvaz Jundishapur University of Medical Sciences, to ensure a representative sample of the MS population in the region. This center serves as a referral hub for MS patients in Khuzestan province, with initial diagnoses made by neurologists. Patient evaluations at the center are overseen by neurologists, with final diagnoses based on clinical and radiological evidence. Data accuracy was ensured through cross-referencing multiple databases and validation by trained personnel.
Patient information, including age, age at diagnosis, disease duration, initial symptoms, the Expanded Disability Status Scale (EDSS), gender, education, employment status, ethnicity, marital status, and MS subtype, was extracted from the Khuzestan MS Center database. This database covered records from the association's establishment in 2005 to the end of 2019. Prevalence estimates of MS by county in 2019 were calculated using population data adjusted for natural population growth rates between census years.
The data regarding the number of dusty days, average daily temperature (in degrees Celsius), and daily wind speed (in meters per second) were obtained from the Environmental Protection Agency and the Meteorological Organization of Khuzestan province. These datasets were collected from the beginning to the end of 2019 for various counties within the province. Subsequently, the daily values of these variables were averaged annually for each county. Finally, the prevalence rates of MS for 2019 were correlated with the annual averages of the number of dusty days, temperature, and wind speed across the 27 counties.
3.1. Statistical Analysis
Descriptive statistics, including frequency, mean, standard deviation, median, and interquartile range (IQR), were reported. A multi-pollutant generalized additive model (GAM) was employed to estimate the relative risk (RR) of the effects of dusty days, temperature, and wind speed on the prevalence of MS. Generalized additive models represent a powerful class of models for capturing nonlinear effects of continuous quantitative variables in regression models where the response follows an exponential family distribution (19). These models are an extension of generalized linear models (GLMs) and offer a high degree of flexibility. Due to their ability to model nonlinear trends, GAMs have been widely used in many studies (20-22). In fact, GAMs allow the integration of variables in a non-parametric manner using smooth functions, such as splines (19).
The assumptions for using GAMs are as follows:
1. Independence of observations.
2. The response variable should follow a distribution from the exponential family (e.g., normal, binomial, Poisson).
3. The effects of the predictors are assumed to be additive and are modeled through separate smooth functions for each predictor.
4. The smoothing functions (e.g., splines) used in the model should be smooth.
5. The link function that connects the predictors to the response variable must be appropriate (23).
The standard formulation of a GAM (24) can be stated as follows:
- Y is the response variable.
- E[Y] is the expected value of the response variable.
- g is the link function that relates the expected value of Y to the predictors (for example, normal, binomial, or Poisson distributions).
- β0 is the intercept.
- fi (Xi) are smooth functions applied to the predictor variables Xi (e.g., splines).
- n represents the number of predictor variables.
In this ecological study, the response variable consisted of count data (the number of MS cases per 100,000 people), thus following a Poisson distribution. However, due to the unequal mean and variance in the response variable data, the quasi-Poisson regression model was utilized.
The GAM model provides the capability to detect nonlinear relationships between variables. In this study, a semiparametric regression approach was employed, which models the effects of the predictors using a combination of parametric and nonparametric regression techniques while retaining essential data characteristics. This method allows for more precise calculation of coefficients and the effects of independent variables, resulting in a better fit to the data through the use of nonlinear and flexible smoothing functions, such as splines. The GAM utilized penalized cubic regression splines to model the nonlinear relationships between predictors and the response variable (23).
The model used in this study is outlined as follows:
Where:
- Bj is the estimated coefficient of covariate variable j.
- Sj is a smooth function of the covariate "dusty days" (j = 1, …., q).
- q is the number of spline functions in the GAM model.
In this analysis, the unit of measurement used was the increase of one unit in the level of all independent variables, which was applied to calculate the RR for the outcome (the prevalence of MS per 100,000 people). The unconnected lines above and below the curves represent the 95% confidence interval. The 'mgcv' package (23) in R software version 3.4.3 was employed for the GAM analysis. A significance level of less than 0.05 was considered.
4. Results
In this study, a total of 2,676 patients with MS were registered at the Khuzestan MS Center from its establishment in 2005 until the end of December 2019, all of whom were included in the analysis. The mean age of the MS patients was 31.40 ± 8.94 years. The sex ratio (female to male) was 3.05, indicating that approximately two-thirds of the patients were female. The mean age at MS diagnosis was 29.19 ± 9.16 years. Notably, 75% of the patients were diagnosed before the age of 35.75. Of the patients, 30% were female, with the majority holding associate's and bachelor's degrees (35.10%), being married (62.10%), and ethnically belonging to the Lor group (34.50%). Regarding the type of MS, relapsing-remitting MS (RRMS) was the most prevalent, while only 3.5% had primary progressive MS (PPMS) and 4.9% had secondary progressive MS (SPMS) (Table 1).
| Variables | Values |
|---|---|
| Age (y) | |
| Mean ± SD | 31.40 ± 8.94 |
| Median ± IQR | 30.00 ± 11 |
| EDSS | |
| Mean ± SD | 1.857 ± 1.7846 |
| Median ± IQR | 1.500 ± 1.5 |
| Duration of MS disease (y) | |
| Mean ± SD | 1.95 ± 3.658 |
| Median ± IQR | 0.0 ± 2 |
| Age-incidence of MS | |
| Mean ± SD | 29.19 ± 9.16 |
| Median ± IQR | 28.00 ± 12 |
| Time between onset of symptoms and definitive diagnosis (months) | |
| Mean ± SD | 14.84 ± 31.601 |
| Median ± IQR | 2.00 ± 12 |
| Gender | |
| Female | 2015 (75.3) |
| Male | 661 (24.7) |
| The first sensory sign | |
| No | 1684 (62.9) |
| Yes | 992 (37.1) |
| The first motor sign | |
| No | 2214 (82.7) |
| Yes | 462 (17.3) |
| The first equilibrium sign | |
| No | 2332 (87.1) |
| Yes | 344 (12.9) |
| The first visual sign | |
| No | 1663 (62.1) |
| Yes | 1013 (37.9) |
| The first urinary disorder sign | |
| No | 2603 (97.3) |
| Yes | 73 (2.7) |
| The first fatigue sign | |
| No | 2550 (95.3) |
| Yes | 126 (4.7) |
| Education | |
| Illiterate | 144 (5.4) |
| Under diploma | 679 (25.4) |
| Diploma | 782 (29.2) |
| Post-diploma and bachelor's degree | 940 (35.1) |
| Master’s degree and higher | 131 (4.9) |
| Job | |
| Employed | 697 (26.0) |
| Unemployed | 749 (28.0) |
| Disabled | 91 (3.4) |
| Housewife | 1139 (42.6) |
| Race | |
| Persian | 891 (33.3) |
| Turkish | 61 (2.3) |
| Kurd | 63 (2.4) |
| Lor | 922 (34.5) |
| Arab | 704 (26.3) |
| Others | 35 (1.3) |
| Marital status | |
| Single | 931 (34.8) |
| Married | 1661 (62.1) |
| Divorced | 62 (2.3) |
| Widower/widow | 22 (0.8) |
| MS type | |
| RR-MS | 2041 (76.3) |
| PP-MS | 93 (3.5) |
| SP-MS | 132 (4.9) |
| RP-MS | 25 (0.9) |
| DEVIC | 67 (2.5) |
| CIS | 318 (11.9) |
Abbreviations: EDSS, Expanded Disability Status Scale; IQR, interquartile range.
a Values are expressed as No. (%), unless otherwise indicated.
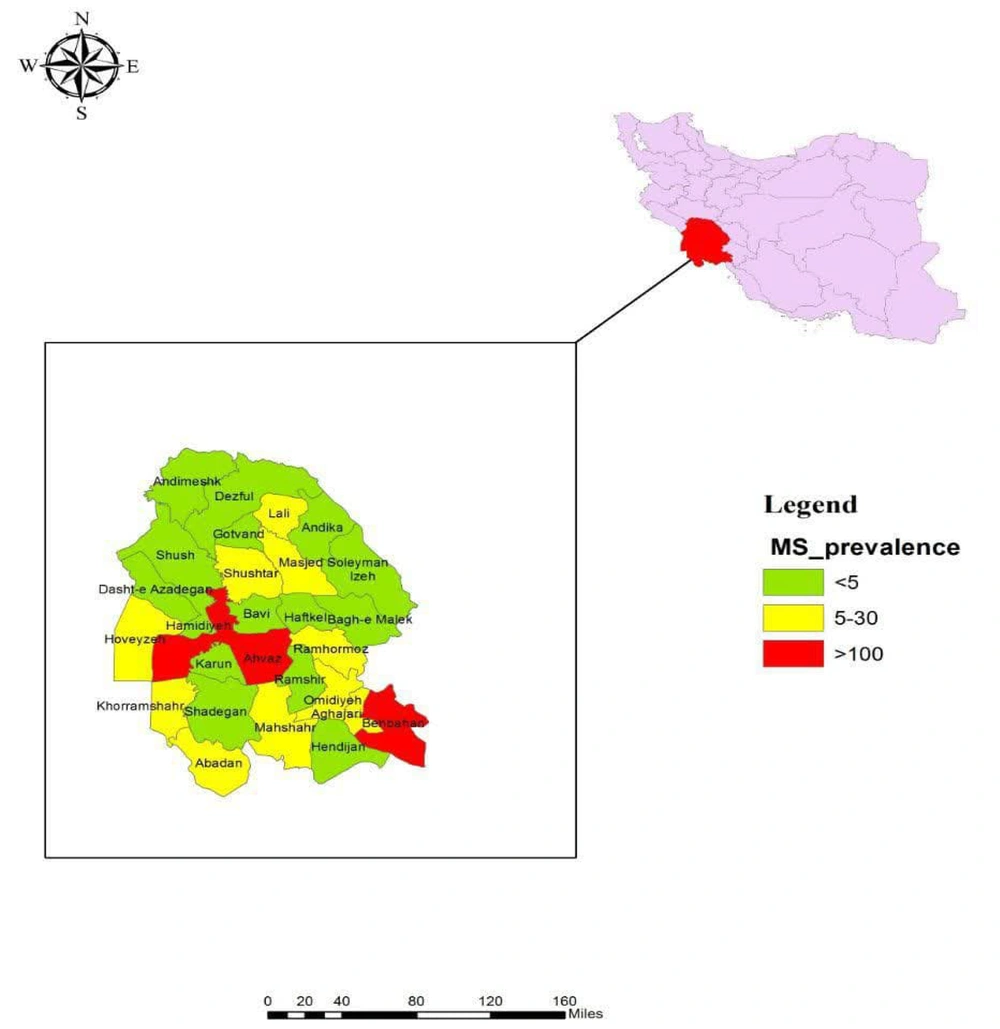
Overall, in 2019, the average annual prevalence of MS in Khuzestan province was 42 per 100,000 people. Table 2 presents the distribution of MS prevalence according to geographical classification in Khuzestan province. As shown, the lowest prevalence of MS was observed in Bavi county, with a rate of 4 cases per 100,000 individuals. Conversely, the highest prevalence of MS occurred in Behbahan county, with a rate of 112 cases per 100,000 individuals, followed by Ahvaz county with a rate of 109 cases per 100,000 individuals in 2019 (Table 2, Figure 1). Additionally, the mean temperature, number of dusty days, and wind speed (expressed in kilometers per hour) across Khuzestan province in 2019 were recorded as 25.25 ± 2.16 (ranging from 20 to 30), 47.26 ± 15.7 (ranging from 18 to 78), and 39.37 ± 13.13 (ranging from 14.30 to 60.80), respectively.
| Names | MS Prevalence per 100,000 Individuals | Temperature (°C) | Dusty Days | Wind Speed (km/h) |
|---|---|---|---|---|
| Bavi | 4 | 24 | 78 | 24.2 |
| Karun | 12 | 25.2 | 66 | 30.1 |
| Haftgel | 14 | 27 | 46 | 48.5 |
| Andika | 15 | 27 | 46 | 48.5 |
| Hamidiyeh | 17 | 23 | 25 | 56.74 |
| Shadegan | 17 | 25 | 50 | 46.5 |
| Gotvand | 20 | 20 | 24 | 43.8 |
| Shush | 20 | 22 | 46 | 32.1 |
| Ramshir | 20 | 25 | 60 | 30.1 |
| Dasht-e Azadegan | 22 | 28 | 36 | 24.9 |
| Dezful | 29 | 24 | 18 | 60.8 |
| Izeh | 33 | 24 | 78 | 24.2 |
| Hendijan | 34 | 24 | 25 | 56.74 |
| Andimeshk | 34 | 23.9 | 37 | 46.7 |
| Baghmalek | 39 | 30 | 52 | 24.9 |
| Masjedsoleyman | 41 | 26 | 34 | 45.3 |
| Lali | 42 | 27.5 | 52 | 24.9 |
| Bandar-e Mahshahr | 54 | 27.4 | 30 | 43.5 |
| Abadan | 55 | 27.5 | 59 | 55.9 |
| Khorramshahr | 55 | 24.5 | 60 | 57.3 |
| Omidiyeh | 55 | 25 | 49 | 46.5 |
| Shushtar | 66 | 25 | 49 | 46.5 |
| Ramhormoz | 66 | 24.5 | 49 | 43.8 |
| Aghajari | 68 | 28.4 | 36 | 14.3 |
| Hoveyzeh | 77 | 22.7 | 54 | 21.7 |
| Ahvaz | 109 | 25.2 | 66 | 30.1 |
| Behbahan | 112 | 26 | 51 | 34.5 |
Abbreviation: MS, multiple sclerosis.
The results of the GAM model indicated that there was no statistically significant association between average temperature and the prevalence of MS (P-value = 0.994). However, a significant positive statistical association was observed between the number of dusty days and the prevalence of MS in Khuzestan province (P-value < 0.001). For each unit increase in the number of dusty days, the prevalence of MS increased by 11 cases per 100,000 individuals (RR = 1.113, P-value < 0.001). Conversely, a significant negative statistical association was observed between average wind speed and the prevalence of MS. For each unit increase in average wind speed, the prevalence of MS decreased by 1 case per 100,000 individuals (RR = 0.999, P-value < 0.001) (Table 3).
Abbreviation: RR, relative risk.
a The P-value is significant (P-value < 0.05).
5. Discussion
Recent studies in Iran have investigated the role of air pollution as a significant factor in the occurrence of autoimmune diseases, including MS (7, 25, 26). A study conducted between 2008 and 2016 in Isfahan found that the level of the Air Quality Index (AQI) was associated with the EDSS of MS patients, suggesting that air pollution may play a role in both the severity of MS (27).
Outside of Iran, Jeanjean et al. (28), in Strasbourg, France, conducted a study between 2000 and 2009 based on meteorological parameters, school holidays, and public holidays. They reported that the impact of PM10 on the onset and recurrence of MS was more significant in the winter season than in the summer season, which is consistent with our study's findings that an increase in the number of dusty days is associated with an increase in the prevalence of MS in Khuzestan province. Similar positive associations between particulate matter air pollution and MS have been reported in other studies conducted in Iran, Italy, and Finland (12, 13, 29).
In a cross-sectional study by Tateo et al. in Padua, Italy, the results indicated a strong association between the prevalence of MS and annual PM2.5 concentration levels (r = 0.81, P < 0.001). Regression analysis further confirmed this relationship, revealing that MS cases were significantly associated with average PM2.5 levels (β = 0.11, P < 0.001) (30). In the United States, a case-control study by Lavery et al. showed that particulate matter (PM2.5) (OR = 3.96, 95% CI: 1.42 - 11.1, P < 0.01), along with three other criteria pollutants (SO₂, CO, and lead), was significantly associated with an increased likelihood of pediatric MS (31).
A case-crossover study by Jeanjean et al. in Paris, France, indicated that elevated NO2 and PM10 exposure were linked to an increased risk of MS relapse during the cold season (OR = 1.08, 95% CI: 1.03 - 1.14; OR = 1.06, 95% CI: 1.01 - 1.11, respectively). Moreover, a significant association was found between ozone (O3) exposure and MS relapse risk during the hot season (OR = 1.16, 95% CI: 1.07 - 1.25) (28). Similarly, a cross-sectional study by Ashtari et al. in Isfahan, Iran, revealed that the AQI level correlated with the degree of complete remission following the first attack of MS, with an odds ratio of 1.005 (95% CI: 1.004 - 1.006) (27). In an ecological study by Heydarpour et al. conducted from 2003 to 2013 in Tehran, Iran, the findings indicated that MS cases exhibited a clustered distribution throughout the city. Additionally, a significant difference in exposure to pollutants such as PM10, SO2, NO2, and NOx (P < 0.001) was found when comparing MS cases to control subjects (12).
Collectively, these studies underscore the significant association between particulate matter and MS, suggesting that exposure to air pollution, particularly particulate matter, may contribute to the prevalence and severity of this neurological disease.
Among the environmental factors considered, some studies have focused on the role of temperature, sunlight, and other meteorological parameters. In this regard, Vojinovic et al., in the city of Nis, Serbia, conducted a 5-year study and reported a significant positive correlation between total cloudiness and daily cloudiness with the number of MS relapses, although no relationship was found between disease activity and direct sunlight exposure in terms of hours, days, or sunny months (32). In another study, Tremlett in southern Tasmania, Australia, observed a lower frequency of MS relapses in summer compared to winter, based on data collected from January 2002 to April 2005 (33). Similarly, Ma and Zhang in China, from January 2002 to December 2012, associated MS onset/relapse with geographical latitude, temperature, and sunlight intensity. They observed the highest onset/relapse of MS in winter (134 cases) and the lowest in summer (97 cases). According to them, environmental factors, especially sun exposure, had the most significant impact on disease onset and relapse, while gender, age of onset, and disease duration were not significant (34). On the other hand, in a study by Abella-Corral et al. conducted from 1997 to 2002, a significant difference in the prevalence of MS was reported between summer (high prevalence, peaking in June) and winter (low prevalence, lowest in December) (35).
Some studies have examined the seasonal and monthly correlation between meteorological parameters and the prevalence and relapse of MS. For instance, Fundora-Hernandez et al. in Cuba, between April 2004 and November 2007, reported the highest frequency of MS prevalence in the second quarter of the year, from April to June, attributing it to the transition from winter to summer and the variable thermal oscillation during that period (36). Additionally, Ogawa et al. in Japan, in 2004, investigated the correlation between temperature and MS exacerbation. They divided the 12 months of the year into 6 groups based on the monthly average temperature and found that the total number of disease attacks was higher in the warmest months (July and August) and the coldest months (January and February) compared to other months (37).
Recently, in a population-based study involving three countries with high MS prevalence (Canada, Italy, and Norway), behavior and lifestyle related to sunlight exposure in summer and winter were examined. The results indicated that the risk of MS was higher among individuals who spent more time indoors during their childhood and protected themselves from the sun during the limited periods they spent outdoors. However, this protection did not affect the risk of MS among individuals who spent a considerable amount of time outdoors in the summer and a moderate amount of time outdoors in the winter (38). These findings contrast with the results of our study regarding the role of temperature in the prevalence of MS. Our study did not observe a significant statistical correlation between average temperature and MS prevalence. Comparing our findings with those of the aforementioned studies is somewhat challenging, as they used different meteorological criteria related to the prevalence or exacerbation of MS, likely due to the substantial differences in climate between Khuzestan province and the other regions studied.
A significant limitation of this study is its reliance on aggregated data, which restricts the applicability of the findings to individual cases. Additionally, the exposure levels captured by air pollution and meteorological stations may not accurately reflect personal exposure. Since the study was ecological and population-based, it was not possible to account for individual confounders such as smoking, education, and other relevant variables. One of the strengths of this study is that, at the time it was conducted, no prior research had explored the connection between exposure to dusty days, temperature, wind speed, and the prevalence of MS in Khuzestan province, Iran.
Our study results showed that an increase in dusty days is associated with a higher prevalence of MS, highlighting the need for further investigation into the environmental factors affecting MS. These findings also suggest that public health interventions in Khuzestan province should prioritize monitoring and managing environmental factors, particularly during dusty seasons. Finally, future research should explore the mechanisms by which environmental factors, especially particulate matter, influence MS risk and consider longitudinal studies to assess changes over time.