1. Background
Obsessive-compulsive disorder (OCD) is a chronic mental illness characterized by repetitive behaviors (compulsions) and unwanted thoughts (obsessions). While repetitive actions are common in daily life, individuals with OCD experience these behaviors and thoughts more frequently and intensely, significantly impacting their lives almost constantly (1, 2). Obsessive-compulsive disorder is the fourth most common mental disorder globally (3). Its estimated lifetime prevalence is 2.3%, with a range of 1.1 - 3.3%, varying by factors such as location, age, and other demographic variables (1, 4). Individuals with OCD may display various symptom categories, including persistent unwanted or religious thoughts, frequent hoarding, symmetry, frequent checking, fixation with contamination and cleaning methods, and ordering. These symptoms and behaviors may fluctuate, become irregular, or change in manifestation over the course of the disorder (5, 6).
Research in the past few decades has significantly expanded our understanding of the phenomenology and treatment of OCD. Numerous mechanisms and factors contribute to the etiology of OCD, leading to its chronic nature. Recent years have seen increased attention on the neuropsychology of OCD. This disorder is linked to neuropsychological impairments, particularly in areas such as attention, memory, and executive functions (7, 8). Based on the neurological mechanisms implicated in OCD, it is believed that the disorder involves deficiencies in two primary inhibitory processes: (1) Cognitive inhibition process: Associated with obsessive symptoms; (2) behavioral inhibition process: Associated with compulsive symptoms (9).
Deficiencies in these two inhibitory systems explain not only the cognitive and behavioral symptoms of OCD but also many neuropsychological deficits observed in this disorder, such as impairments in attention, memory, planning, and decision-making (7, 9).
There is substantial evidence linking OCD to brain dysfunction and cognitive abnormalities (10). Neuropsychological studies consistently reveal that individuals with OCD perform worse compared to other populations and clinical samples (11).
Underlying factors often accelerate the progression of diseases, and in the case of OCD, rumination appears to be a significant contributing factor. Memory interference can lead to rumination, which is frequently observed in individuals with OCD and generalized anxiety disorder (12). Research confirms that rumination plays a role in exacerbating psychological disturbances (13). This style of thinking is also prevalent in several emotional disorders, including depression, OCD, generalized anxiety disorder, and post-traumatic stress disorder (14).
The clinical literature on OCD highlights cognitive behavioral therapy (CBT), particularly in the form of exposure and response prevention (ERP), as the first-line treatment for this disorder (15). However, despite significant advances in OCD treatment, some patients either do not respond to treatment or show minimal improvement (16). While certain patients experience partial recovery, residual symptoms persist, leading to significant functional impairment and a reduced quality of life. Additionally, some patients are unwilling or unable to tolerate the anxiety associated with ERP, while others abandon or refuse the treatment altogether (17). Consequently, the treatment of OCD remains a challenging area in psychological therapy.
The ongoing search for more effective treatment methods continues to drive extensive and varied research in this field. Among the emerging therapeutic techniques is brain stimulation, which has gained attention in recent years as a safe and effective non-invasive intervention. In particular, transcranial direct current stimulation (tDCS) has shown promising results (18).
Harika-Germaneau et al. (19) studied the efficacy of 10 sessions of tDCS in treating treatment-resistant OCD. In this study, 80 treatment-resistant outpatients with OCD were randomly assigned to receive either active or sham tDCS. Patients were assessed at baseline, at the end of the treatment, and during one- and three-month follow-ups. A significant interaction between time and treatment was observed. However, the primary endpoint — change in Yale-Brown Obsessive Compulsive Scale (Y-BOCS) scores after two weeks—was not achieved. In contrast, the secondary endpoint, which measured changes in Y-BOCS scores after three months, was successfully met (19). These findings underscore the importance of studying the placebo effect in tDCS interventions and the necessity of long-term follow-up to evaluate the optimal effects of the treatment. Similarly, Brunelin et al. (20) concluded that despite methodological limitations and variability in stimulation parameters, tDCS appears to be a promising tool for alleviating OCD symptoms.
Obsessive-compulsive disorder is a complex disorder, recognized as the tenth leading cause of disability among patients. It is also one of the most prevalent mental health conditions in Iran, with an estimated prevalence ranging from 11.7% to 43.2% (21). Given that conventional psychological and pharmacological treatments do not consistently result in recovery and that a significant proportion of patients are resistant to these interventions, researchers have increasingly focused on novel treatment approaches. These include neurobiological methods that offer the potential for neurological adjustments (22).
2. Objectives
Considering the limited research on the efficacy of such interventions, this study was designed to evaluate the effectiveness of tDCS in improving executive functions, reducing the severity of obsessive-compulsive symptoms, and addressing rumination in individuals with OCD symptoms.
3. Methods
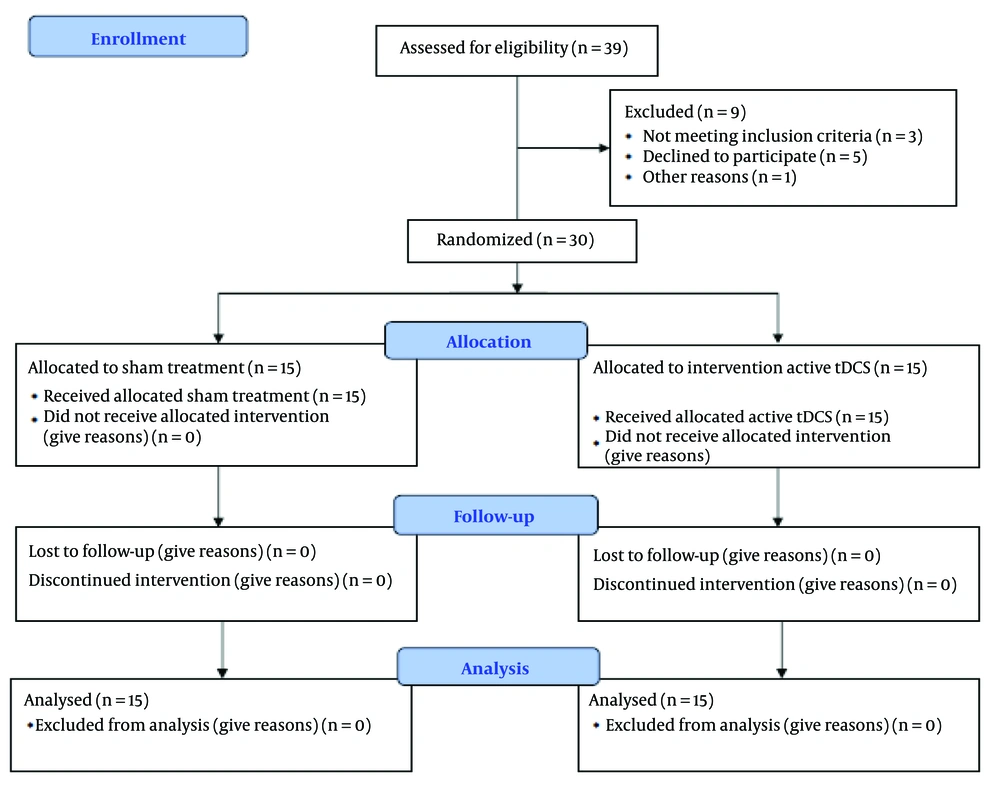
The current research falls under the category of applied research in terms of its goal and experimental designs regarding its data collection method. This study was a randomized clinical trial with a pre-and post-test design, consisting of two groups. The statistical population included all individuals with obsessive-compulsive symptoms who visited the psychiatric clinic of Golestan Hospital and the Counseling Center of Jundishapur University of Medical Sciences in Ahvaz during 2022 - 2023. Thirty participants meeting the inclusion and exclusion criteria were randomly assigned to two groups. In this double-blind, controlled, randomized study, 30 patients with obsessive-compulsive symptoms (determined by a score higher than 11 on the Maudsley Questionnaire) were assigned to receive 10 sessions (one session per day) of either active tDCS (2 mA) or sham treatment, each lasting 20 minutes. Of these, 15 patients received active tDCS, and 15 received sham treatment.
3.1. Inclusion Criteria
Participants had to obtain a score higher than 11 on the Maudsley Obsessional-Compulsive Questionnaire (MOCI), be willing to participate in the research, not receive simultaneous psychological treatments such as group therapy, cognitive-behavioral therapies, mindfulness, muscle relaxation, or stress management, be aged between 18 and 60 years, and complete a written consent form.
3.2. Exclusion Criteria
Individuals were excluded if they had a history of psychotic disorders, serious head injuries, substance abuse, acute suicidal ideation, or implanted metal or devices in the brain. Pregnant women were also excluded. Additionally, patients previously exposed to tDCS could not participate. Other exclusion criteria included unavailability during the research period, severe physical illnesses such as cancer, the use of a cardiac pacemaker, severe organic brain disorders based on patient and family history or psychiatric examination, the presence of evident bipolar or psychotic symptoms, and absence from tDCS sessions.
After coordinating and obtaining the code of ethics from Ahvaz University (IR.AJUMS.HGOLESTAN.REC.1400.152) and the clinical trial code (IRCT20220220054069N1), the researcher entered the research environment with permission. The research was conducted at the Golestan Hospital Psychiatry Clinic and the Counseling Center of Ahvaz University of Medical Sciences. Before the preliminary evaluations, all conditions for participating in the research were explained to the applicants, allowing them to proceed with the evaluation process if they wished. Full explanations about the purpose of the project were provided to participants before obtaining consent and completing the questionnaire. After explaining the research, its conditions, and other ethical matters, participants were asked to complete the informed consent form.
Regarding sample selection, it should be noted that in experimental methods, each subgroup should consist of at least 15 people to ensure the sample is a true representative of the community and that the research maintains high external validity (23, 24). A total of 38 individuals were evaluated for eligibility. Eight people did not participate, leaving a total of 30 participants who completed the study. Of these, 15 patients received active tDCS, and 15 patients received sham treatment. In this randomized double-blind controlled study, 30 patients with obsessive-compulsive symptoms were assigned to receive 10 sessions (one session per day) of active tDCS (2 mA) or sham treatment. Pre-test evaluations were conducted, and after the treatment concluded, re-evaluations were performed.
It should be noted that no costs were imposed on the participants in this study. At the beginning of the research and after the intervention, all participants were assessed using the MOCI, the Executive Functions Questionnaire, the Y-BOCS, and the Mistake Rumination Scale. As shown in CONSORT Figure 1, the sample size was based on previous research indicating a high effect size according to Cohen's criteria for the studied treatments. With a test power of 0.80 and a significance level of 0.95, 15 participants were allocated to each group.
Regarding sample selection, it is important to reiterate that in experimental methods, each subgroup should consist of at least 15 participants to ensure the sample is representative of the community and the research has high external validity (23, 24). To analyze the findings, descriptive statistics, analysis of covariance, independent t-tests, and chi-square tests were employed. All analyses were performed using SPSS-23 software.
3.3. Measures
The MOCI: This is a 30-item tool with true and false responses. It provides a total score and seven sub-scores. The cutoff point for this scale is eleven. In Iran, the reliability of this instrument was reported as 0.85 using the test-retest method, and the reliability coefficient for the entire test was 0.84 (25).
Executive Functions Questionnaire: This is a 35-item scale used to measure executive functions. Participants respond using a five-point Likert scale ranging from never to always. This scale has demonstrated good psychometric properties (26). The reliability of the subscales, calculated using the omega coefficient, ranged from 0.82 to 0.94 (26).
3.3.1. Yale-Brown Obsessive Compulsive Scale
Developed by Goodman et al. (27), this scale is used to determine the symptoms and severity of obsessive-compulsive symptoms. It contains 10 items - 5 items evaluate obsessive thoughts, and 5 items assess compulsions. Responses are scored on a 5-point Likert scale ranging from very severe (4) to subclinical (0). The total score ranges from 0 to 40, indicating the intensity of obsession and compulsion (27). In Iran, findings showed significant internal consistency for both the symptom and severity subscales, with coefficients of 0.97 and 0.95, respectively, and a test-retest reliability of 0.99 (28).
3.3.2. The Mistake Rumination Scale
This seven-item scale measures the tendency to ruminate about past personal mistakes. It has a single-factor structure and high internal consistency. Responses range from not at all to very high. The scale has demonstrated good psychometric properties (29, 30), with a Cronbach's alpha coefficient of 0.93 (30).
3.3.3. Transcranial Direct Current Stimulation
The tDCS is a non-invasive brain stimulation technique that applies a weak direct current (1 - 2 mA) between two electrodes placed on the scalp. Neurophysiological studies indicate that the polarity of the electrode and the intensity of the current determine the effects of tDCS. Specifically, anodal tDCS may increase the excitability of the cerebral cortex near the electrode, while cathodal tDCS may reduce excitability. The effects of tDCS extend beyond the targeted electrode area, influencing cortical networks and subcortical regions connected to the target areas. It is worth noting that the details of microscopic brain processes and the molecular mechanisms underlying tDCS effects on brain function remain largely unknown (31). This study evaluated the efficacy of tDCS, with the cathode applied to the orbitofrontal cortex (OFC) and the anode applied to the right cerebellum, in reducing OCD symptoms.
4. Results
4.1. Description of the Sample
In this research, 30 subjects with an age range of 17 - 51 years and an average age of 36.50 ± 8.94 participated. Among these 30 subjects, 15 were in the intervention group and 15 in the control group. Eight participants (26.7%) were male, and 22 participants (73.3%) were female. Nine participants (30.00%) were single, 19 were married (63.33%), and 2 (6.66%) were divorced. Regarding the educational qualifications of the participants: Four people (13.3%) had a middle school education, 1 person (3.3%) had a diploma, 2 people (6.7%) had postgraduate education, 15 people (50.0%) had a bachelor's degree, and 8 people (26.7%) had postgraduate education or higher.
Eighteen participants (60.0%) were employed, while 12 participants (40.0%) were unemployed. Five participants (16.7%) reported a history of drug use, while 25 participants (83.3%) reported no history of drug use. Two participants (6.6%) reported a medical history, while 28 participants (93.3%) reported no medical history.
The results of the chi-square test showed no significant difference in the education and gender variables between the two groups (P > 0.05). However, the results of the independent t-test indicated that the average age in the control group (39.93 ± 7.29) was higher than in the intervention group (33.06 ± 9.33), and this difference was statistically significant (P < 0.05).
As shown in Table 1, the mean post-test scores compared to the pre-test scores in the active tDCS group demonstrated a greater decrease in MOCI, Y-BOCS, and rumination variables compared to the sham treatment group. Additionally, there was a greater increase in the executive functions variable in the active tDCS group compared to the sham treatment group.
| Variables and Groups | Pre-test | Post-test |
|---|---|---|
| MOCI | ||
| Active tDCS | 22.40 ± 2.09 | 14.46 ± 3.06 |
| Sham treatment | 21.53 ± 1.80 | 21.66 ± 1.54 |
| Y-BOCS | ||
| Active tDCS | 22.33 ± 7.72 | 14.40 ± 2.99 |
| Sham treatment | 20.33 ± 6.89 | 18.13 ± 6.99 |
| Mistake Rumination Scale | ||
| Active tDCS | 20.93 ± 4.16 | 11.46 ± 2.13 |
| Sham treatment | 19.13 ± 5.34 | 18.26 ± 4.89 |
| Executive Functions Questionnaire | ||
| Active tDCS | 86.40 ± 14.38 | 102.86 ± 8.40 |
| Sham treatment | 83.06 ± 16.61 | 86.93 ± 15.54 |
Abbreviations: MOCI, Maudsley Obsessional-Compulsive Questionnaire; tDCS, transcranial direct current stimulation; Y-BOCS, Yale-Brown Obsessive Compulsive Scale.
The assumptions of the covariance analysis method, including independence of observations, homogeneity of the variance-covariance matrix across groups, homogeneity of regression slopes, homogeneity of variances, and normality of the dependent variable distribution, were appropriate and observed.
According to Table 2, the effectiveness of tDCS on obsessive thoughts and compulsions in MOCI was significant at the 0.001 level, with an effect size of 0.752. This indicates that tDCS accounts for 75.27% of the variance in changes in obsessive thoughts and compulsions in MOCI from pre-test to post-test. The power of the statistical test in this analysis is 1.000, reflecting the high ability of the test to detect significant differences.
| Variable | Source of Change | SS | df | F | P-Value | Effect Size | Statistical Power |
|---|---|---|---|---|---|---|---|
| MOCI | Group | 416.271 | 1 | 82.02 | 0.001 | 0.752 | 1.000 |
Abbreviation: MOCI, Maudsley Obsessional-Compulsive Questionnaire.
According to Table 3, the effectiveness of tDCS on obsessive thoughts and compulsions in the Y-BOCS was significant at the 0.001 level, with an effect size of 0.412. This means that tDCS explains 41.2% of the variance in changes in obsessive thoughts and compulsions in the Y-BOCS from pre-test to post-test. The power of the statistical test in this analysis is 0.987, demonstrating the high power of the test in identifying significant differences.
| Variable | Source of Change | SS | df | F | P-Value | Effect Size | Statistical Power |
|---|---|---|---|---|---|---|---|
| Y-BOCS | Group | 179.627 | 1 | 18.89 | 0.001 | 0.412 | 0.987 |
Abbreviation: Y-BOCS, Yale-Brown Obsessive Compulsive Scale.
According to Table 4, the effectiveness of tDCS on rumination was significant at the 0.001 level, with an effect size of 0.706. This suggests that tDCS accounts for 70.6% of the variance in rumination changes from pre-test to post-test. The power of the statistical test in this analysis is 1.000, indicating the high sensitivity of the test to detect significant differences.
| Variable | Source of Change | SS | df | F | P-Value | Effect Size | Statistical Power |
|---|---|---|---|---|---|---|---|
| Mistake rumination | Group | 444.07 | 1 | 64.96 | 0.001 | 0.706 | 1.000 |
According to Table 5, the effectiveness of tDCS on executive functions was significant at the 0.001 level, with an effect size of 0.418. This indicates that tDCS explains 41.8% of the variance in changes in executive functions from pre-test to post-test. The power of the statistical test in this analysis is 0.987, confirming the high power of the test in discovering significant differences.
| Variable | Source of Change | SS | df | F | P-Value | Effect Size | Statistical Power |
|---|---|---|---|---|---|---|---|
| Executive Functions Questionnaire | Group | 1444.79 | 1 | 19.38 | 0.001 | 0.418 | 0.989 |
5. Discussion
Obsessive-compulsive disorder is a chronic and treatment-resistant neuropsychological disease that often develops during childhood and adolescence, leading to significant long-term problems in a person's life. This disorder involves uncontrollable thoughts that compel individuals to repeat specific actions, causing disruptions in daily functioning (32). Despite advances in patient management, approximately 30 - 60% of patients show only a partial response to current medications or experience no improvement (33). Increasing evidence suggests that the neurobiological substrates of OCD include abnormal activity and connectivity in the orbitofronto-striato-pallido-thalamus network, with heightened activity in the OFC, cingulate gyrus, and caudate (34). Consequently, this research aimed to evaluate the effectiveness of tDCS on executive functions, the severity of obsessive-compulsive symptoms, and rumination in individuals with obsessive-compulsive symptoms.
The results of our study comparing the two groups demonstrated that the mean post-test scores, compared to the pre-test scores, in the active tDCS group showed a greater reduction in obsessive-compulsive symptoms and rumination. Additionally, there was a greater improvement in executive functions in the active tDCS group compared to the sham treatment group, with a significant difference between the two groups. Fineberg et al. (32) reported that tDCS treatment improves cognitive control in individuals with OCD.
Asadollahzadeh Shamkhal et al. (35) indicated that tDCS induces changes in brain complexity in individuals with contamination-related OCD by influencing neuronal interactions and balancing neuronal activity. Bation et al. (18) showed that, despite significant acute effects, tDCS was not effective in achieving long-term symptom reduction in patients with treatment-resistant OCD. In another study (36), tDCS, with the anode placed on the right cerebellum and the cathode placed on the left OFC, was found to be a safe, favorable, and appropriate approach to reducing OCD symptoms in treatment-resistant patients.
Brunelin et al. (20) conducted a systematic study investigating the effects of tDCS on OCD reviewing 12 studies involving a total of 77 patients (20). The findings indicated that, despite methodological limitations and heterogeneity in stimulation parameters, tDCS appears to be a promising tool for reducing obsessive-compulsive symptoms, as well as co-occurring depression and anxiety, in patients with treatment-resistant OCD.
Transcranial direct current stimulation enables simultaneous stimulation of various regions and modulation of different brain areas involved in cortico-subcortical loops (37). It has been suggested that tDCS may effectively reduce symptoms in patients with treatment-resistant OCD (38), although the optimal target sites and stimulation parameters remain a topic of debate.
One possible explanation for its effectiveness is that tDCS helps regulate brain activity, leading to a reduction in obsessive-compulsive symptoms. Previous studies have shown that tDCS can improve symptoms by influencing the interactions and dynamics of brain neurons. It is concluded here that tDCS, by modulating brain neuron activity and bringing it closer to a normal state, causes changes in the complexity of brain activity in individuals with obsessive-compulsive symptoms.
In explaining these findings, it can be posited that individuals with OCD experience disturbances in executive functions. By stimulating the frontal lobe, tDCS improves executive functions in these individuals, thereby enhancing their cognitive performance. Furthermore, tDCS's ability to modulate neuroplasticity, specifically by increasing cortical excitability in the left dorsolateral prefrontal cortex (DLPFC) and decreasing it in the right DLPFC, contributes to the improvement of obsessive-compulsive symptoms.
One of the limitations of the present research is the sample size. Although comparable to other studies in this field, it can be considered relatively small, particularly given the heterogeneity in OCD. Further studies should focus on factors that can enhance the clinical efficacy of tDCS. First, inducing activity during the stimulation of targeted neural networks is crucial for achieving neurobiological and clinical effects, as tDCS primarily acts by enhancing neural plasticity (39) and learning (40). Using a greater number of sessions over a longer period may be necessary to achieve clinically significant outcomes. The optimal placement of electrodes in OCD remains a challenging issue. Neuroimaging studies can only infer correlation, not causation, which limits the understanding of specific stimulation targets.
Another limitation is the lack of access to a sufficient sample size to examine and compare stimulation protocols with multiple current intensities. Due to time constraints, it was not possible to include a follow-up phase. Additionally, the generalizability of the results is limited. Therefore, caution should be exercised when applying these findings to non-experimental conditions and other clinical groups.
Future research should be conducted with larger sample sizes and include follow-up procedures. The effectiveness of stimulation protocols using multiple current intensities and different electrode sizes should also be investigated. Furthermore, the protocol used in this study should be tested on other clinical groups and common psychiatric disorders. This study did not account for the effect of medications, which should be considered in future research. Follow-up periods should also be included to assess the long-term stability of tDCS effects.
5.1. Conclusions
Transcranial direct current stimulation appears to have a significant effect on obsessive-compulsive symptoms, rumination, and executive functions in people with obsessive-compulsive symptoms. Using a greater number of tDCS sessions over a longer duration may be necessary to achieve clinically meaningful results. Moreover, determining the optimal electrode placement in OCD remains an important and complex issue that requires further investigation.