1. Background
Iron deficiency is the most prevalent micronutrient deficiency worldwide, affecting numerous patients, particularly pregnant women (1-3). This deficiency is increasingly common in pregnant women, with a prevalence of 40% in the first trimester of pregnancy. Iron deficiency can cause significant health issues for patients, especially pregnant mothers, and can adversely affect fetal development (4). Evidence indicates that iron deficiency during pregnancy can lead to various disorders, particularly neurological disorders in fetuses (5).
The prevalence of iron deficiency in pregnant women varies, with approximately 22% in developed countries. The maternal mortality rate due to iron deficiency in these regions is also 20%. Globally, around 32 million pregnant women are iron deficient, predominantly in South Asia and Africa (6, 7). During pregnancy, the fetus receives nutrients through the placenta, and iron deficiency in mothers can often be asymptomatic, with effects manifesting in infants post-birth (8-10).
The precise causes of iron deficiency in pregnant mothers are not fully understood, but several factors are implicated. Poor nutrition, excessive bleeding, and certain medications are among the primary contributors to iron deficiency (10-12). Maternal iron deficiency, with or without anemia, particularly when ferritin levels are below 12 μg/L, can negatively impact fetal iron status and the growth and function of major organ systems, including the heart, skeletal muscles, brain, and gastrointestinal tract (13-16).
A critical issue concerning iron deficiency is its occurrence in pregnant women. Some women exhibit lower than normal serum iron levels without clinical symptoms, complicating the differentiation between anemic and non-anemic pregnant women. The absence of anemia symptoms can delay diagnosis and disrupt fetal development during pregnancy. Humayun et al. demonstrated that diet significantly influences iron deficiency in pregnant women, regardless of anemia status (17). Another study found that iron deficiency is more severe in pregnant women without anemia, sometimes necessitating major interventions (18-20). Eweis et al. reported a 72% prevalence of iron deficiency among pregnant women, primarily due to inadequate fruit and meat consumption, excessive tea intake, and lack of iron supplementation (7). Ribot et al. found iron deficiency prevalence rates of 3.8% in the first trimester and 21.5% in the third trimester, raising concerns about maternal and fetal health (21).
2. Objectives
Despite the focus on iron deficiency anemia during pregnancy, iron deficiency without anemia is less frequently addressed. Therefore, this study aims to investigate the prevalence of iron deficiency in mothers without anemia during the first trimester of pregnancy.
3. Methods
3.1. Study Design
This study is a prospective cohort study conducted on 876 pregnant women who presented to Mahdieh Hospital in 2022. All participants were enrolled after confirming they met the inclusion criteria.
3.2. Participants
Following approval from the Vice President of Research and obtaining the ethics code from Shahid Beheshti University of Medical Sciences, the study commenced. The study population included pregnant women without anemia, with a gestational age between 1 and 14 weeks. Patient selection was random and based on availability.
3.3. Inclusion and Exclusion Criteria
Inclusion criteria were as follows: Pregnant women aged 15 years and older, gestational age between 1 and 14 weeks, no history of anemia before pregnancy [hemoglobin (Hb) ≥ 11 mg/dL] in the first trimester, and absence of hematological diseases, chronic liver and kidney diseases, diabetes, heart disorders, inflammatory bowel diseases, and malignant neoplasms. Additional criteria included dietary uniformity, exclusion of low-income patients, and exclusion of strictly vegetarian or meat-eating patients.
Exclusion criteria included a history of hereditary and acquired blood diseases, thalassemia, and failure to perform necessary tests.
3.4. Procedure
In this study, 876 pregnant women over the age of 15 who presented to Mahdieh Hospital in Tehran during 2022, and who did not have anemia (Hb < 11 mg/dL) in the first trimester, were interviewed regarding study participation. After explaining the study objectives and obtaining written consent, a patient profile form was completed, which included age, height, weight, BMI, education, economic status, parity, history of abnormal bleeding before pregnancy, and timing of previous delivery. Current pregnancy details were recorded. Blood samples were collected to determine Hb, hematocrit (HCT), and ferritin levels, and results were documented. A ferritin level < 30 ng/dL was considered indicative of iron deficiency, based on guidelines in women’s health texts (22). Confounding factors included the presence of inflammation, treatment with iron supplements, infection, and absence of chronic anemia.
3.5. Measuring and Data Collection
Hemoglobin and HCT levels were measured using a Sysmex X10 device. For ferritin measurement, plasma samples were separated from blood using a refrigerated centrifuge at 3000 rpm for 10 minutes. Ferritin levels were then measured by radioimmunoassay using a relevant kit (Orion Diagnostic, Finland). Demographic data were collected from patient records, while laboratory indices were obtained from complete blood count (CBC) results.
3.6. Statistical Analysis and Sample Size
Data were entered into SPSS version 24 software for analysis. Descriptive statistics, including frequency distribution tables and graphs, were used to describe the variables under study. The chi-square test was employed to compare categorical variables. The normality of the data was assessed using the Kolmogorov-Smirnov test. Due to the non-normal distribution of the data, the relationship between continuous variables was examined using the Mann-Whitney test. Logistic regression was utilized to control for confounding factors and to examine their effects. With a study power of 90% and a confidence level of 95%, the sample size was calculated as 876 patients based on the following formula:
4. Results
Table 1 presents the demographic data of the patients. The results indicate that the mean age of the patients was 30.88 ± 6.65 years, and the mean BMI was 29.46 ± 4.62 kg/m2. A total of 549 patients (62.7%) were multiparous. Regarding dietary habits, the majority of patients were omnivorous (97.1%). Additionally, it was observed that most patients did not have a history of abnormal uterine bleeding (AUB) or abortion.
| Variables and Categories | Mean ± SD or No (%) |
|---|---|
| Age (y) | 30.88 ± 6.65 |
| Weight (kg) | 73.60 ± 12.35 |
| Height (cm) | 158.27 ± 5.87 |
| BMI corrected (kg/m2) | 29.46 ± 4.62 |
| Hb (gr/dL) | 12.43 ± 1.09 |
| HCT (%) | 36.29 ± 3.91 |
| Ferritin (ng/dL) | 38.85 ± 36.96 |
| Parity | |
| Primiparous | 327 (37.3) |
| Multiparous | 549 (62.7) |
| Abortion | |
| No | 625 (71.3) |
| Yes | 251 (28.7) |
| AUB | |
| No | 858 (97.9) |
| Yes | 18 (2.1) |
| Income | |
| Low | 34 (3.9) |
| Middle | 781 (89.2) |
| High | 61 (7) |
| Diet | |
| Omnivorous | 851 (97.1) |
| Vegetarian | 25 (2.8) |
Descriptive Information of Patients
4.1. Evaluation of Demographic Information Based on Ferritin Levels
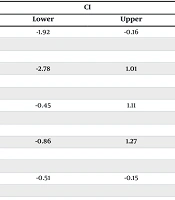
The results demonstrated that the mean age and Hb levels were significantly higher in patients with ferritin levels ≥ 30 ng/dL (P < 0.05). On the other hand, no correlation was observed between weight, height and BMI with ferritin level in patients (P > 0.05) (Table 2).
| Variables | Mean ± SD | CI | P-Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age (y) | -1.92 | -0.16 | 0.02 | |
| < 30 | 30.38 ± 6.68 | |||
| ≥ 30 | 31.42 ± 6.58 | |||
| Weight (kg) | -2.78 | 1.01 | 0.36 | |
| < 30 | 73.19 ± 12.17 | |||
| ≥ 30 | 74.07 ± 12.56 | |||
| Height (cm) | -0.45 | 1.11 | 0.40 | |
| < 30 | 158.43 ± 5.95 | |||
| ≥ 30 | 158.09 ± 5.59 | |||
| BMI (kg/m2) | -0.86 | 1.27 | 0.44 | |
| < 30 | 29.98 ± 4.81 | |||
| ≥ 30 | 29.78 ± 4.91 | |||
| Hb (gr/dL) | -0.51 | -0.15 | < 0.001 | |
| < 30 | 12.27 ± 1.00 | |||
| ≥ 30 | 12.60 ± 1.16 | |||
Evaluation of Demographical Information Based on Ferritin
4.2. Evaluation of Patient Information According to Ferritin Levels
Patient data were evaluated based on ferritin levels. The analysis revealed no significant relationship between parity, education, income, dietary habits, and AUB with ferritin levels in patients (P > 0.05). However, a significant relationship was found between abortion incidence and ferritin levels. Specifically, the incidence of abortion was higher in patients with ferritin levels ≥ 30 ng/dL (P = 0.01) (Table 3).
| Variables | Ferritin (ng/dL) | P-Value | |
|---|---|---|---|
| < 30 | ≥ 30 | ||
| Parity | 0.06 | ||
| Primiparous | 44 (9.6) | 52 (12.4) | |
| Multiparous | 412 (90.4) | 368 (87.6) | |
| Abortion | 0.01 | ||
| No | 341 (74.8) | 284 (67.6) | |
| Yes | 115 (25.2) | 136 (32.4) | |
| Education | 0.85 | ||
| Illiterate | 13 (2.9) | 14 (3.3) | |
| Elementary | 52 (11.4) | 49 (11.7) | |
| Guidance | 87 (19.1) | 68 (16.2) | |
| High school | 257 (56.4) | 245 (58.3) | |
| University | 47 (10.3) | 44 (10.5) | |
| Income | 0.98 | ||
| Low | 1 (2.3) | 1 (1.9) | |
| Middle | 40 (90.9) | 47 (90.4) | |
| High | 3 (6.8) | 4 (7.7) | |
| Diet | 0.27 | ||
| Omnivorous | 43 (97.7) | 52 (100) | |
| Vegetarian | 1 (2.3) | 0 (0) | |
| AUB | 0.51 | ||
| No | 448 (98.2) | 410 (97.6) | |
| Yes | 8 (1.8) | 10 (2.4) | |
Evaluation of Information of Patients According to the Ferritin a
4.3. Logistic Regression Analysis
The table below presents the logistic regression analysis conducted to evaluate the effect of various factors on anemia. The results indicated that, among the factors investigated, only Hb demonstrated a statistically significant relationship (Table 4). By incorporating confounding factors into the regression model, including Hb, HCT, and dietary habits, their effects were controlled. The analysis revealed that only the Hb factor remained statistically significant (Table 5).
| Variables | Beta | t | P-Value | 95% CI | |
|---|---|---|---|---|---|
| Down | UP | ||||
| Constant | - | 0.095 | 0.925 | -19.768 | 21.747 |
| Age | 0.053 | 0.458 | 0.648 | -0.013 | 0.020 |
| Weight | 0.341 | 0.214 | 0.831 | -0.127 | 0.158 |
| Height | -0.101 | -0.135 | 0.893 | -0.140 | 0.122 |
| BMI | -0.891 | -0.545 | 0.587 | -0.478 | 0.272 |
| Parity | -0.217 | -1.538 | 0.128 | -0.506 | 0.065 |
| Abortion | 0.035 | 0.316 | 0.752 | -0.223 | 0.307 |
| Hb | 0.295 | 2.307 | 0.023 | 0.021 | 0.282 |
| HCT | 0.001 | 0.004 | 0.997 | -0.049 | 0.049 |
| Diet | 0.058 | 0.516 | 0.607 | -0.222 | 0.378 |
| AUB | 0.018 | 0.138 | 0.891 | -0.607 | 0.697 |
Logistic Regression Analysis
| Variables | B | S.E. | Wald | df | Sig. | Exp (B) | 95% CI for Exp (B) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Step 1a | ||||||||
| Hb | 0.258 | 0.091 | 8.013 | 1 | 0.005 | 1.294 | 1.083 | 1.547 |
| HCT | -0.024 | 0.030 | 0.635 | 1 | 0.426 | 0.977 | 0.921 | 1.035 |
| Food regime | 0.211 | 0.470 | 0.200 | 1 | 0.654 | 1.234 | 0.491 | 3.104 |
| Constant | -2.564 | 0.911 | 7.912 | 1 | 0.005 | 0.077 | - | - |
Logistic Regression Analysis for Confounding Factors
5. Discussion
Iron deficiency is a common issue faced by pregnant women. While it can cause short- and long-term problems for the mother, it also affects fetal development (5). Issues such as premature birth, low birth weight, and neurological disorders are major concerns for infants born to mothers with iron deficiency. Many pregnant women do not exhibit symptoms of iron deficiency, making early diagnosis in the first trimester crucial for effective management (23).
In this study, a relationship was found between patient age and Hb levels with ferritin levels. Specifically, the mean age and Hb were higher in patients with ferritin levels ≥ 30 ng/dL (P < 0.05). In the study by Resseguier et al., it was demonstrated that Hb levels were lower in pregnant women with iron deficiency compared to the control group. Additionally, no relationship was found between iron deficiency and patient age, which contrasts with the findings of the present study. However, consistent with our results, no correlation was observed between BMI, weight, and height with iron deficiency. Furthermore, the incidence of iron deficiency was higher in low-income families (6).
Hajianfar et al. identified a relationship between patient nutrition and the incidence of iron deficiency, showing that reduced iron absorption is associated with maternal and neonatal complications. They also found that iron deficiency was not related to patient height and weight but was related to Hb levels (24). Abbas et al. reported that iron deficiency was not associated with age, BMI, parity, and patient age, aligning with our study (25). Differences in results may be attributed to the varying sample sizes and participant characteristics across studies.
Overall, demographic factors such as age, BMI, and parity were not associated with iron deficiency in this study and similar research. However, due to their significance in patient physiology, these factors may be useful for future screening. In the present study, no relationship was found between diet, income, and AUB with ferritin levels. Conversely, the abortion rate was significantly higher in patients with ferritin levels ≥ 30 ng/dL. Zhao et al. found a significant relationship between iron deficiency and the number of past abortions, with a higher prevalence of abortion in affected patients (26). Other studies reported no relationship between miscarriage and iron deficiency, with similar abortion rates in iron-deficient and normal pregnant women (27, 28). Differences in results may arise from varying study groupings, such as comparisons between pregnant and non-pregnant women or anemic and non-anemic pregnant women, whereas our study focused solely on non-anemic pregnant women.
In this study, no association was found between diet and iron deficiency in non-anemic pregnant women (P > 0.05). Evidence suggests that diet significantly impacts vitamin and mineral absorption. Dietary regulation is crucial for fetal development in pregnant women. Previous studies indicate that a meat-inclusive diet may prevent iron deficiency during pregnancy, while vegetarians have a higher incidence of iron deficiency (29-31).
5.1. Conclusions
In summary, Hb levels and abortion history appear to influence iron deficiency in pregnant women without anemia, whereas nutrition, income, and ferritin levels do not seem to have an effect. Based on these findings, future studies should focus on patient nutrition and include a larger sample size. Utilizing Hb measurements may aid in the timely diagnosis of iron deficiency. Additionally, iron supplementation and the consumption of iron-rich foods during pregnancy can help manage iron levels in pregnant women.
5.2. Study Limitations
This study has several limitations:
(1) The sample size was relatively small; future studies should include a larger cohort.
(2) Participants were recruited from a single educational center; more comprehensive results could be obtained by including multiple centers.
(3) The impact of patients' dietary habits on iron deficiency should be further evaluated.
(4) The majority of participants were of Afghan ethnicity, which may limit the generalizability of the findings.