1. Background
Ovarian aging is a physiological process that, despite the decrease in the number of eggs, can also affect their fertility. Given that ovarian aging develops over time and the healing and treatment process requires a long time, it is considered a chronic disease (1). Reduction of ovules in follicles can cause problems such as infertility in patients. The decrease in ovarian reserve that can occur as a result of aging leads to a disorder in spontaneous fertility as well as a decrease in the success of artificial insemination methods (2). Therefore, the use of therapeutic strategies to increase ovarian reserve can be effective in the fertility success of patients (3, 4).
One of these strategies is the use of platelet-rich plasma (PRP). Based on established evidence, PRP contains many growth factors, including platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), epidermal growth factor (EGF), insulin-like growth factor-1 (IGF-1), and many other growth factors (1, 5-8). These factors interact with each other and with other factors in controlling the physiological processes of the cell, including inflammation, oxidative stress, angiogenesis, cell proliferation, and differentiation (9, 10). Using the PRP method can be a safe method for patients because it is prepared from the patient's own blood and does not contain any foreign substances. On the other hand, this method is cost-effective and does not require special equipment. The growth factors in PRP each have their own unique functions and play an important role in the regeneration of cells (11).
In systematic reviews and before-and-after retrospective studies, the main body of evidence regarding the effectiveness of PRP injection in improving ovarian reserve is formed. Despite the conflicting results regarding the improvement of ovarian reserve, most of the evidence emphasizes the necessity of conducting more prospective studies in this emerging field (12-14). Accordingly, Melo et al. showed that the use of PRP before an ART cycle can increase ovarian reserve and increase fertility rates in patients (15). However, in another study, the results showed that after PRP, only 8% of patients had a live birth, indicating that the use of other methods in addition to PRP is needed to improve fertility (16).
The emergence of autologous PRP therapy reflects a breakthrough approach that is showing promising results. The PRP injections are available as intrauterine and intraovarian injections. Previous evidence suggests that intrauterine injections may be less effective than intraovarian injections in patients with reduced ovarian reserve. However, comprehensive evidence is lacking (15). At present, there are very few studies on this issue. Therefore, the present study aims to investigate the effectiveness of intraovarian injection of PRP in women with infertility related to reduced ovarian reserve and poor response to ovulation stimulation in vacuum IVF.
2. Objectives
In this study, we investigated the effect of PRP injection on hormone levels, including follicle-stimulating hormone (FSH), LH, and anti-Müllerian hormone (AMH), as well as ovule and embryo number in infertile patients.
3. Methods
3.1. Design Study
The current research is a semi-experimental study conducted on 17 infertile women referred to Nikan Hospital between 2021 and 2022 who were candidates for PRP therapy due to reduced ovarian reserve. The 17 patients participating in the study were evaluated in a single group before and after PRP treatment. After obtaining approval from the Vice President of Research and the ethics code from Shahid Beheshti University of Medical Sciences, the study commenced. This study also has a clinical trial registration code: IRCT20160722029027N14.
3.2. Patients Selection
Initially, 35 patients were enrolled in the study. Ten patients were excluded due to not meeting the inclusion criteria. Of the remaining 25 patients, eight were excluded during follow-up, leaving 17 patients. Therefore, this study was conducted on 17 patients who received the PRP intervention.
3.3. Inclusion and Exclusion Criteria
Inclusion criteria were infertile women under 42 years of age with diminished ovarian reserve, indicated by AMH < 1 ng/mL or antral follicle count (AFC) < 5, and infertile women with a poor ovarian response, evidenced by a history of recovering fewer than 5 oocytes in IVF. Exclusion criteria included infertility due to endometriosis and pelvic inflammatory disease (PID), hyperandrogenism and polycystic ovary syndrome (PCOS), anatomical disorders, male factor infertility, and platelet deficiency or bleeding disorders.
3.4. Procedure
For the women included in the study, demographic characteristics and records related to previous pregnancies were extracted from their medical records. To perform the intervention, patients underwent ultrasound on the 1st to 3rd day of menstruation, ensuring the absence of follicles larger than 10 mm, endometrial thickness below 5 mm, and ovulation. Treatment with gonadotropin Pergoveris (recombinant gonadotropin, Cinnal pen, Cinagen Co) at 300 IU was injected daily, along with human menotropin gonadotropin (HMG) at 2 ampules per day. When the developing follicle reached a size of 12 - 14 mm, a GnRH antagonist (cetrotide 0.25 mg/day) was started subcutaneously. This injection continued until the day of administration of 250 μg Ovitrelle recombinant HCG. Ovulation was induced using 250 μg Ovitrelle recombinant HCG when the dominant follicle reached 18 mm. Transvaginal oocyte retrieval was performed within 34 to 36 hours after the injection of 250 μg Ovitrelle recombinant HCG under ultrasound guidance. On the day of ovulation, after ovarian puncture, 3 cc of PRP containing platelets 4 - 5 times more than serum was injected.
In the third menstrual cycle, after checking serum levels of FSH and AMH and the presence of at least one AFC in the ultrasound, the patients were again placed in the cycle of ovulation stimulation. The improvement of the ovarian response was determined by evaluating the parameters of the number of retrieved oocytes, the number of obtained embryos, and the serum levels of AMH, FSH, and the number of AFC in ultrasound.
3.5. Statistical Analysis
The Kolmogorov-Smirnov test was used to assess the normality of the variables before and after PRP injection. The results indicated that these variables followed a normal distribution, allowing for the use of a paired t-test to compare the average serum levels of AMH, FSH, and AFC, as well as the number of oocytes retrieved in IVF and the number of embryos formed in IVF before and after PRP intraovarian injection. A significance level of less than 0.05 was considered statistically significant.
4. Results
4.1. Demographical Information of Patients
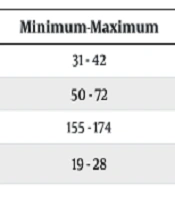
The following table presents the descriptive information about the patients. The average age, weight, height, and Body Mass Index (BMI) of the patients were 36.94 ± 3.76 years, 59.76 ± 6.32 kg, 161.94 ± 5.09 cm, and 22.36 ± 2.89 kg/m2, respectively (Table 1).
| Variables | Mean ± SD | Minimum-Maximum |
|---|---|---|
| Age (y) | 36.94 ± 3.76 | 31 - 42 |
| Weight (kg) | 59.76 ± 6.32 | 50 - 72 |
| Height (cm) | 161.94 ± 5.09 | 155 - 174 |
| BMI (kg/m2) | 22.36 ± 2.89 | 19 - 28 |
Demographical Information of Patients
4.2. Evaluation of Hormone Levels Before and After the Platelet-Rich Plasma
The results showed that the average FSH level was higher before the PRP injection and decreased significantly after the PRP injection (P = 0.014). The average AMH level increased after the PRP injection compared to before, but this difference was not statistically significant (P = 0.661). Additionally, the average AFC increased significantly after the PRP injection compared to before (P = 0.009) (Table 2).
| Variables | PRP (Mean ± SD) | Effect Size | 95% Confidence Interval | P-Value | ||
|---|---|---|---|---|---|---|
| Before | After | Low | Up | |||
| FSH (mlu/mL) | 11.61 ± 3.08 | 10.31 ± 2.65 | 0.657 | 0.133 | 1.165 | 0.014 |
| AMH (mlu/mL) | 0.59 ± 0.38 | 0.61 ± 0.33 | -0.106 | -0.570 | 0.361 | 0.661 |
| AFC (n) | 2.88 ± 1.57 | 3.82 ± 1.94 | -0.708 | -1.224 | -0.175 | 0.009 |
Measuring Hormone Level Before and After Platelet-Rich Plasma
4.3. Evaluation of the Number of Ovules and Embryos Obtained
Based on the results, it was found that the average number of oocytes retrieved increased significantly after the PRP injection compared to before (P = 0.017). Additionally, the average number of embryos obtained also increased significantly after the PRP injection compared to before (P = 0.018) (Table 3).
| Variables | PRP (Mean ± SD) | Effect Size | 95% Confidence Interval | P-Value | ||
|---|---|---|---|---|---|---|
| Before | After | Low | Up | |||
| Ovules | 5.06 ± 2.63 | 7.23 ± 4.11 | -0.629 | -1.133 | -0.109 | 0.017 |
| Embryo | 3 ± 2.14 | 5 ± 3.03 | -0.700 | -1.264 | -0.116 | 0.018 |
Number Ovule and Embryo Obtained Related Before and After Platelet-Rich Plasma
5. Discussion
Platelet-rich plasma is currently one of the most common regenerative agents in clinical practice, known for releasing growth factors and proteins that have beneficial effects on wound healing and regeneration processes (16). Platelets in PRP are activated by stimuli, leading to the release of growth factors and cytokines from the granules. These factors modulate the proliferation and regeneration of cells through the regulation of molecular pathways (17). Evidence suggests that activated platelets release growth factors and cytokines that regulate cellular processes such as proliferation, differentiation, and angiogenesis through signaling pathways including mTOR, JAK/STAT, and AKT. Degradation of the extracellular matrix by growth factors leads to angiogenesis in ovarian cells, contributing to an increase in ovarian reserve (18).
In this study, the average BMI of patients was 22.36 ± 2.89, and the average age was 36.94 ± 3.76. Previous evidence indicates that patients with a BMI higher than the normal range are less responsive to PRP. A study by Hernandez-Melchor et al. showed that PRP use in patients can be associated with an increased frequency of clinical pregnancy. However, further studies revealed that fertility rates were lower in obese patients, who did not respond well to PRP (19). Another factor is the age of the patients. Recent studies have shown that age is a key factor in patients' response to PRP, with younger patients experiencing greater improvement rates (20).
The present study was designed to investigate the effectiveness of PRP intraovarian injection on improving ovarian reserve in infertile women with decreased ovarian reserve. The results showed that the average AFC, the average number of oocytes retrieved in IVF, and the average number of embryos obtained in IVF significantly increased after intraovarian injection of PRP (P < 0.05). Additionally, the average FSH level significantly decreased after PRP intraovarian injection (P < 0.05). However, the average AMH level did not change significantly (P > 0.05).
In a meta-analysis by Li et al., the results indicated that intraovarian injection of PRP had significant therapeutic effects in increasing AMH levels, AFC, and the number of oocytes and embryos (P < 0.05). The data of patients before and 2 months after treatment were compared, showing that PRP injection effectively reduced FSH levels, increased AMH levels, and increased the number of antral follicles, oocytes, and embryos (P < 0.05) (21). Additionally, when the dose of PRP injected into each ovary was ≥ 4 ml, a significant correlation was observed with improvements in AFC, oocytes, and embryos (21).
In another study, PRP injection in primary ovarian insufficiency (POI) patients led to an increase in AMH and AFC, with no change in FSH. Out of 313 patients, only 8 had live births or sustained implantation (1). Aflatoonian et al. showed that LH and FSH levels decreased two months after PRP injection, while estradiol (E2) and AMH levels increased one month after injection but decreased in the second month. These results were not consistent with the present study, possibly due to the timing of factor measurements after PRP injection (22).
Previous studies have shown that PRP injection in infertile patients can cause changes in signaling pathways and genes. Specifically, PRP injection has been shown to increase AMH levels in patients. Increasing AMH can inhibit NF-kB activity and prevent inflammation. Additionally, AMH activates the mTOR pathway, promoting follicle proliferation and preventing apoptosis (23, 24). Anti-Müllerian hormone is a key hormone in regulating follicle metabolism, so PRP injection can improve AMH levels and increase ovarian reserve (25).
The PRP contains a series of growth factors, cytokines, and other macromolecules, each playing an important role in the physiological processes of cells (26). Therefore, the use of PRP can be effective in regulating hormones and improving ovarian function (27, 28). Previous studies have also shown that growth factors can regulate gene expression through signaling pathways. Gene regulation can enhance the structure and function of follicles and increase fertility. Consequently, PRP can regulate uterine thickness, potentially increasing the success of IVF and live births (18, 29). The study by Coksuer et al. showed that clinical pregnancy and live birth rates were higher in the group injected with PRP compared to the control group (30).
This study has several limitations. The number of patients studied was limited due to the specific indications for PRP and the presence of confounding factors, such as PCOS. Additionally, patients were collected from only one center, as the study was conducted under the supervision of Shahid Beheshti University of Medical Sciences, limiting the infertility centers involved. Another limitation is that only one group participated in this study, with pre- and post-intervention assessments conducted within the same group. Future studies should include a control group alongside the intervention group for more comprehensive evaluation.
5.1. Conclusions
Based on this study, PRP intraovarian injection appears to improve ovarian reserve and increase the number of oocytes and embryos. It also regulates sex hormones, potentially leading to increased fertility rates. The use of PRP in infertile patients can be effective in improving fertility rates and preparing the endometrium for embryo implantation. Additionally, since PRP contains growth factors, it can aid in repairing damaged cells. Improving the levels of sex hormones (FSH, LH, and AMH) through PRP injection can enhance the number and quality of oocytes. Sex hormones, along with growth factors, can promote the regeneration and proliferation of ovarian tissue, leading to increased fertility in patients.