1. Background
Hyperthyroidism, which refers to the excessive production of thyroid hormones in an individual, usually requires a well-planned diagnosis and treatment to prevent systemic complications, such as those seen in cardiovascular and metabolic abnormalities (1). Biomarker tests, such as thyroid-stimulating hormone (TSH), free thyroxine (fT4), and free triiodothyronine (fT3), are essential in managing hyperthyroidism, as they guide treatment decisions and monitor response during the course of treatment (2). Considering that regional healthcare practices and genetic factors influence the presentation and response to treatment, it is vital to understand how biomarker profiles and clinical outcomes interplay in Iraq (3). It has been observed that treatments like carbimazole are widely used to suppress hormone synthesis, but differing strategies in dosing and patient adherence can make optimal care more difficult to achieve (4). The study is limited to Iraqi patients as it attempts to explore how biomarker dynamics intersect with local healthcare practices.
A major area of ongoing debate in the literature concerns the dynamics of thyroid biomarkers in response to antithyroid therapy, particularly carbimazole, and the extent to which these markers reliably predict clinical remission or relapse (5). For instance, several studies from Western populations have demonstrated that early normalization of TSH and fT3 following antithyroid therapy is associated with a lower risk of relapse and better long-term outcomes. In contrast, research from Asian and Middle Eastern cohorts has reported cases where TSH remains suppressed despite biochemical euthyroid status, or where fT4 and fT3 normalization does not correlate with symptomatic improvement. Moreover, while some investigators have found that higher baseline fT4 levels predict increased relapse risk after therapy, others have observed no such association, suggesting that genetic, environmental, or healthcare practice differences may play a modifying role (6, 7).
Many things have improved around the world, but one of the major gaps still exists regarding the use of biomarker data in the Iraqi healthcare system. Although research has clarified the general pharmacodynamic effects of carbimazole (8), its real performance in settings where patients differ from this demographic has not been established worldwide. For example, a study in 2020 demonstrated that higher baseline fT4 levels are significantly associated with the possibility of relapse (9), but a similar situation does not apply to Iraqi cohorts. This research innovatively examines biomarker test characteristics against health practices with a cohort of Iraqi patients with hyperthyroidism. It compares 25 carbimazole-compliant patients with 25 patients on no treatment for three months to assess the impact of routine therapy on TSH, fT4, and fT3 levels measured through enzyme-linked immunosorbent assay (ELISA) and discern barriers to effective treatment (10). Within this work, the aims include establishing the diagnostic potential of these biomarkers, gauging the effect of carbimazole on hormone normalization, and establishing how test results will guide clinical management at Al-Sader Teaching Hospital. Therefore, this dual focus on biochemical and practical vectors creates a novel paradigm for optimizing regional hyperthyroidism management.
The article is divided into five parts. First, it presents the pathophysiology of hyperthyroidism and the role of biomarkers in diagnosis and monitoring, emphasizing regional-specific challenges in Iraq (11). Second, the methodology section details the ELISA-based quantification of TSH, fT4, and fT3, emphasizing technical approaches that may be employed to avoid biotin-related mismeasurements (10, 12). The third part, results, compares biomarker trajectories between treated and untreated groups and correlates them with patient demographics and adherence patterns. The fourth part contextualizes the findings within the reality of the Iraqi healthcare system and proposes pathways through which test utilization and awareness among patients might be enhanced. Lastly, recommendations are made with respect to the incorporation of biomarkers into clinical work processes, corroborated by global and regional evidence (13).
2. Objectives
The present study aimed to evaluate the drug carbimazole — an antithyroid drug — and its effect on therapeutic conservation in patients diagnosed with hyperthyroidism. Accordingly, this study will assess carbimazole’s ability to restore patients’ euthyroid state, which will be measured through changes in selected thyroid function biomarkers —TSH, fT4, and fT3 — within a stated therapeutic period. Therefore, by comparing treatment responses in patients undergoing carbimazole therapy to those not undergoing therapy, the study may bring more clarity to the drug’s role in biochemical normalization and symptom control within routine clinical practice in Iraq (14, 15).
In the long term, this study’s findings are expected to result in evidence-based guidelines regarding clinical decision-making, optimization of treatment strategies, and the overall quality of care administered to hyperthyroid patients in Iraq and other similar health systems (16).
3. Methods
3.1. Study Design
Prospective, controlled observational studies were employed to observe the impact of carbimazole therapy in hyperthyroid patients in this study, conducted at Al-Sader Teaching Hospital, Misan city, Iraq. A total of 50 adult patients aged 20 - 68 years were recruited for the study, having recently been diagnosed with hyperthyroidism based on medical history, clinical examination, and confirmatory blood tests. In addition, 10 healthy controls were selected from the same population, matched for age and sex, with no history of thyroid disease or other significant medical conditions. All participants provided informed consent prior to enrollment.
The patients were divided into two groups: Twenty-five individuals receiving carbimazole treatment and 25 untreated individuals at the time of sampling. Comprehensive demographic and clinical data were collected for all participants, and blood samples were obtained for the measurement of thyroid hormone levels and related biomarkers. Further, to establish biomarker values for comparison, 20 age-matched healthy individuals were assigned as the control group. The hyperthyroid patients were then randomized into two equal groups. Group 1 consisted of 25 patients receiving carbimazole therapy, as directed by their physicians, and were followed up for three months. Group 2 included 25 patients who, of their own volition, did not undergo any antithyroid treatment for the duration of the study but remained under regular observation by their healthcare providers for emergent complications due to untreated hyperthyroidism. From start to finish, both groups underwent routine clinical assessment and laboratory monitoring during the study.
Key biomarkers of thyroid function, including TSH, fT4, and fT3, were estimated at baseline and after the end of the three-month follow-up using standardized ELISA protocols. The study design thus permitted a comparison between biochemical and clinical outcomes in both treated and untreated patients, as well as with healthy controls, thereby determining the therapeutic effect and safety profile of carbimazole in this population. This methodology aligns with the methodologies used to properly assess an antithyroid drug in clinical practice, offering a fair opportunity to map changes in biomarkers to the actual health experience of Iraqi patients with hyperthyroidism (16, 17).
3.2. Biomarker Assessment Using Enzyme-Linked Immunosorbent Assay
Thyroid function in study participants was evaluated using ELISA techniques to quantitatively measure serum levels of TSH, fT4, and fT3. The ELISA is a widely validated immunoassay method for the detection and quantification of specific proteins and hormones in biological samples, offering high specificity and sensitivity for thyroid biomarkers (18).
3.3. Thyroid-Stimulating Hormone Measurement
Serum TSH concentrations were determined using the RayBio® Human TSH ELISA kit, which employs a sandwich ELISA format. In this assay, microplates are pre-coated with an antibody specific to human TSH. Standards and patient samples are added to the wells, allowing TSH present in the samples to bind to the immobilized antibody. After washing to remove unbound substances, a biotinylated anti-human TSH antibody is introduced, followed by horseradish peroxidase (HRP)-conjugated streptavidin. Subsequent addition of a tetramethylbenzidine (TMB) substrate produces a colorimetric reaction proportional to the amount of TSH bound. The reaction is terminated with a stop solution, and absorbance is measured at 450 nm. The TSH concentration in each sample is calculated by comparing the optical density (OD) to a standard curve generated from known concentrations. The assay reference range for TSH was 2.35 - 23.5 ng/dL (19).
3.4. Free Thyroxine Measurement
The fT4 levels were assessed using a competitive ELISA principle (RayBio® Human TSH ELISA Kit). The assay plates were pre-coated with fT4 antigen. During the assay, fT4 in the sample competes with a fixed amount of plate-bound fT4 for binding to a biotinylated detection antibody specific for fT4. After washing to remove excess conjugate and unbound components, an avidin-HRP conjugate is added, followed by incubation. The addition of TMB substrate results in a color change, which is stopped by a stop solution. The intensity of the color, measured at 450 nm ± 2 nm, is inversely proportional to the concentration of fT4 in the sample. The concentration is determined by comparison to a standard curve. The reference range for fT4 was 0.7 - 1.9 ng/dL (20).
3.5. Free Triiodothyronine Measurement
Serum fT3 concentrations were also measured using a competitive ELISA (RayBio® Human TSH ELISA Kit). The assay utilizes plates pre-coated with fT3 antigen. In this format, fT3 in the sample or standard competes with the immobilized fT3 for binding to a biotinylated detection antibody. Following incubation and washing steps to remove unbound substances, an avidin-HRP conjugate is added. The TMB substrate is then introduced, and the reaction is stopped to yield a measurable color change. The OD is read at 450 nm ± 2 nm, and fT3 concentrations are calculated based on a standard curve. The reference range for fT3 was 0.2 - 0.44 ng/dL (21).
All assays were performed according to the manufacturers’ protocols, with appropriate quality controls and calibration standards included in each run. This methodological approach ensures reliable quantification of thyroid biomarkers and supports the robust assessment of treatment effects in hyperthyroidism (22).
4. Results
Untreated hyperthyroidism, marked by a high level of circulating thyroid hormone, can significantly increase risks for sufferers, potentially leading to cardiovascular issues, fragile bones, or metabolic imbalance. Antithyroid drugs, used as the primary means of treating hyperthyroidism, are thus crucial in preventing these complications. Correct estimation of thyroid function is performed through biomarker tests, i.e., TSH, fT4, and fT3; these tests are essential for diagnosing, planning, and monitoring hyperthyroid treatment results.
These biomarkers are responsible for measuring the full effect of thyroid hormones and pituitary feedback on the thyroid. The study centers on Iraqi patients, where genetics, environment, and healthcare access may play a role in the manifestation and outcomes of the disease. Therefore, it is the mechanism by which biomarker levels respond to carbimazole within this population that can develop appropriate treatment modalities. The study will establish whether carbimazole is an efficient way to reach euthyroid status and further investigate its safety in Iraqi patients. By relating the variations in biomarkers from treated and untreated subjects, this research will clarify the benefits and drawbacks of carbimazole therapy. The existing literature on hyperthyroidism management has primarily focused on Western populations, with little attention given to evidence local to Iraq regarding biomarker test characteristics and their relevance to healthcare practices. This study seeks to address this gap. The results from this study should, therefore, be useful in guiding clinical practice parameters and healthcare policy with an evidence-based approach to hyperthyroidism management in Iraq. By generating knowledge about the factors affecting treatment response and potential barriers concerning healthcare access, the research will help improve patient care and outcomes.
The periodic testing of TSH levels is an excellent approach in managing hyperthyroidism, as it allows clinicians to use antithyroid medications, assess their effectiveness, adjust dosages if needed, and monitor potential side effects. This study supports this practice and examines its clinical usefulness as well as practical applicability. In summary, the rationale for this study is anchored in the fact that hyperthyroidism management is critically dependent on biomarker monitoring accuracy and tailoring treatment options to the Iraqi health context. By helping fill in the gaps in localized evidence and guiding clinical practice, the research will help improve hyperthyroidism management and patient outcomes in Iraq.
4.1. Comparison to Healthy Controls and Untreated Groups
The results of this study provide valuable insights into the thyroid hormone profiles of Iraqi patients with hyperthyroidism, comparing them to healthy controls and examining the effects of treatment.
4.1.1. Thyroid-Stimulating Hormone Levels
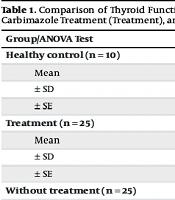
The significant differences observed in TSH levels among the three groups (healthy control, treatment, and without treatment) align with the expected physiological responses in thyroid dysfunction and treatment. The healthy control group exhibited the highest mean TSH level (10.60 ± 5.60), which is consistent with normal thyroid function. In contrast, the markedly suppressed TSH levels in the without-treatment group (1.3840 ± 0.44189) reflect the classic negative feedback mechanism in untreated hyperthyroidism, where excess thyroid hormones suppress pituitary TSH production (Table 1) (23). The intermediate TSH levels in the treatment group (8.51 ± 2.99) suggest a partial restoration of the hypothalamic-pituitary-thyroid axis function with antithyroid therapy, consistent with findings from previous studies (Table 1) (24).
| Group/ANOVA Test | TSH (ng/dL) | FT4 (ng/dL) | FT3 (ng/dL) | P-Value |
|---|---|---|---|---|
| Healthy control (n = 10) | 0.0001 | |||
| Mean | 10.6 | 1.32 | 0.36 | |
| ± SD | 5.60 | 0.37 | 0.06 | |
| ± SE | 1.77 | 0.12 | 0.02 | |
| Treatment (n = 25) | 0.0001 | |||
| Mean | 8.51 | 1.57 | 0.40 | |
| ± SD | 2.99 | 0.37 | 0.08 | |
| ± SE | 0.95 | 0.12 | 0.02 | |
| Without treatment (n = 25) | 0.039 | |||
| Mean | 1.38 | 5.38 | 0.47 | |
| ± SD | 0.44 | 3.20 | 0.13 | |
| ± SE | 0.14 | 1.01 | 0.04 | |
| Total (n = 60) | - | |||
| Mean | 6.83 | 2.75 | 0.41 | |
| ± SD | 5.35 | 2.61 | 0.10 | |
| ± SE | 0.98 | 0.48 | 0.02 |
Comparison of Thyroid Function Parameters (Thyroid-Stimulating Hormone, Free Thyroxine, and Free Triiodothyronine) Among Healthy Controls, Patients Receiving Carbimazole Treatment (Treatment), and Untreated Hyperthyroid Patients with Corresponding ANOVA Test Results Showing Statistical Significance Between Groups
4.1.2. Free Thyroxine Levels
The analysis of fT4 levels reveals a clear distinction between the three groups, with the without-treatment group showing significantly elevated levels (5.38 ± 3.20) compared to the healthy control (1.32 ± 0.37) and treatment groups (1.57 ± 0.376). This pattern is characteristic of overt hyperthyroidism and aligns with established diagnostic criteria (Table 2 and 3) (25). The lower fT4 levels in the treatment group, while still slightly elevated compared to healthy controls, demonstrate the effectiveness of antithyroid medication in reducing thyroid hormone production. This finding is consistent with the expected pharmacological action of drugs like carbimazole, which inhibit thyroid hormone synthesis (6, 26).
Pearson Correlation Coefficients and P-Values for Relationships Among Thyroid Biomarkers (Thyroid-Stimulating Hormone, Free Triiodothyronine, and Free Thyroxine) Within Healthy Controls, Patients Receiving Carbimazole Treatment (Treatment), and Untreated Hyperthyroid Patients
| Groups | TSH | FT3 | FT4 | |||
|---|---|---|---|---|---|---|
| R | P-Value | R | P-Value | R | P-Value | |
| Healthy control and treatment | -0.008 | 0.983 | -0.312 | 0.380 | 0.592 | 0.071 |
| Healthy control and without treatment | 0.477 | 0.163 | -0.096 | 0.791 | -0.081 | 0.824 |
| Treatment and without treatment | -0.115 | 0.752 | 0.376 | 0.284 | -0.152 | 0.674 |
Pearson Correlation Coefficients and P-Values for the Relationships Between Thyroid Biomarkers (Thyroid-Stimulating Hormone, Free Triiodothyronine, and Free Thyroxine) Across Pairs of Study Groups: Healthy Controls, Patients Receiving Carbimazole Treatment (Treatment), and Untreated Hyperthyroid Patients
4.1.3. Free Triiodothyronine Levels
The observed differences in fT3 levels, although less pronounced than those of TSH and fT4, provide additional insights into thyroid function across the groups. The gradual increase from healthy controls (0.360 ± 0.06) to the treatment group (0.40 ± 0.08) and finally to the without-treatment group (0.47 ± 0.13) reflects the spectrum of thyroid hormone production in these conditions. This pattern is consistent with previous research indicating that T3 levels often correlate with the severity of hyperthyroidism (27).
4.2. Correlations Within Groups
The analysis of correlations between thyroid function biomarkers within different groups (healthy control, treatment, and without treatment) provides valuable insights into the complex interplay of thyroid hormones in various physiological states. These findings contribute to our understanding of thyroid function regulation and the effects of hyperthyroidism treatment.
4.3. Healthy Control Group
In the healthy control group, a significant positive correlation was observed between TSH and fT4 (R = 0.637, P = 0.047). This finding is intriguing as it differs from the classical negative feedback relationship typically expected between TSH and thyroid hormones. However, similar observations have been reported in other studies. For instance, Rothacker et al. found that in euthyroid individuals, TSH and fT4 can exhibit a positive log-linear relationship (28). This positive correlation might reflect the individual variability in set points of the hypothalamic-pituitary-thyroid axis, suggesting a more complex regulation than previously thought (29).
4.4. Treatment Group
In patients undergoing treatment for hyperthyroidism, a significant positive correlation was also found between TSH and fT4 (R = 0.664, P = 0.036). This correlation is particularly interesting in the context of treatment. It may indicate that as treatment progresses and fT4 levels begin to normalize, there is a concurrent recovery of TSH production. This finding aligns with studies by Okamura et al., who noted that during the initial phases of antithyroid treatment, the relationship between TSH and thyroid hormones can be variable and may not immediately reflect the negative feedback loop seen in stable euthyroid states (30).
4.5. Without Treatment Group
Interestingly, no significant correlations were observed among TSH, fT3, and fT4 levels in the untreated hyperthyroid group. This lack of correlation could be attributed to the severe disruption of the normal feedback mechanisms in untreated hyperthyroidism. In this state, TSH is often suppressed to very low levels regardless of the degree of T3 and T4 elevation, creating a "floor effect" that obscures any potential correlations (29, 31). This finding is consistent with the pathophysiology of uncontrolled hyperthyroidism, where the negative feedback loop is essentially overwhelmed.
5. Discussion
5.1. Novelty and Originality
The originality of this study lies in its comprehensive examination of thyroid hormone correlations across different states of thyroid function in an Iraqi population. By analyzing these relationships in healthy individuals, treated patients, and those with untreated hyperthyroidism, this research provides a nuanced view of thyroid hormone dynamics that is often overlooked in clinical studies. The positive correlations observed in both the healthy and treatment groups challenge the simplistic view of thyroid hormone regulation and highlight the complexity of the hypothalamic-pituitary-thyroid axis. These findings suggest that the relationship between TSH and thyroid hormones may be more dynamic and individualized than previously recognized, especially in the context of treatment.
Furthermore, the lack of significant correlations in the untreated group provides valuable insight into the severe dysregulation of thyroid function in uncontrolled hyperthyroidism. This observation underscores the importance of prompt diagnosis and treatment to restore normal thyroid hormone relationships.
The study’s focus on an Iraqi population adds to its novelty, as it provides population-specific data that may inform more tailored approaches to diagnosing and managing thyroid disorders in this demographic. The inclusion of both T3 and T4 measurements alongside TSH offers a more comprehensive picture of thyroid status than many previous studies. This research contributes significantly to our understanding of thyroid hormone relationships in different physiological states and treatment conditions. The findings suggest that the interpretation of thyroid function tests should consider the context of the patient’s condition and treatment status. Future research could build on these results to explore the longitudinal changes in thyroid hormone correlations during the course of treatment and investigate potential genetic or environmental factors that may influence these relationships in specific populations.
5.2. Conclusions
Carbimazole was found to be effective in regulating thyroid hormone levels among Iraqi patients who experience hyperthyroidism, as illustrated by significantly elevated TSH levels and lowered fT4 and fT3 levels in subjects who had received treatment, compared with unmedicated individuals. Regular monitoring of these biomarkers, especially TSH, is crucial in managing treatment outcome optimization and complications prevention. The observed correlations of TSH with fT4 in the healthy controls and treated group further illuminate the varied individual differences in the HPT axis. Hence, this research is important in adding insights about tailoring hyperthyroidism management to be more contextually relevant to the local population and health practice. Findings also support the clinical worthiness and practicality of routine TSH testing in enhancing patient outcomes within this context.
5.3. Limitations
This study has several limitations that should be acknowledged. First, the sample size was relatively small, with 25 patients in each hyperthyroid group and 10 healthy controls. This limited sample size may reduce the statistical power of the analyses and restrict the generalizability of the findings to the broader population. Second, the study was conducted at a single center in Misan city, Iraq, which may introduce selection bias and limit the applicability of the results to other regions or healthcare settings with different demographic or clinical characteristics.
Third, while the study focused on key thyroid biomarkers (TSH, fT4, and fT3), other important parameters such as TSH receptor antibodies (TRAbs) were not assessed. The absence of TRAb data limits the ability to fully characterize the autoimmune status of patients and to explore its impact on treatment response and disease prognosis. Fourth, information on patient adherence to carbimazole therapy was based on clinical follow-up and self-report, which may be subject to recall bias or inaccuracies. Additionally, the duration of follow-up was limited to three months, which may not capture long-term treatment outcomes, relapse rates, or late-emerging side effects.
The study also did not perform multivariate analyses to control for potential confounding variables such as age, sex, baseline disease severity, or comorbidities, which could influence biomarker levels and treatment response. Lastly, the lack of a formal sample size calculation and the absence of randomization in group assignment further constrain the robustness of the conclusions. Future studies with larger, multicenter cohorts, longer follow-up periods, inclusion of additional biomarkers (such as TRAb), and more rigorous control of confounding factors are recommended to validate and extend these findings.