1. Background
Irritable bowel syndrome (IBS) is a functional disorder of intestine with major symptoms of chronic abdominal pain and change in bowel habit (1). IBS is one of the most common gastrointestinal (GI) disorders and it is estimated to have a prevalence of about 20% among the developed countries (1, 2). There is no specific laboratory or imaging criteria for its diagnosis and IBS is diagnosed mainly based on Rome criteria and exclusion of other organic disorders (2, 3). IBS could be divided into subgroups based on dominant symptoms (2-4) and bloating is one of its major symptoms among different subgroups (5).
There are many theories about possible pathophysiology of this condition including GI motility disorders (6), central nervous system dysfunction (7), abnormal psychological conditions (8), post infection IBS (9), disturbance of bowel microbiota (10), serotonin pathway disturbance, immune system dysfunction and or mucosal inflammation (11-15). Recent studies have emphasized on the importance of mucosal inflammation and activation of immune system. Based on these theories, symptoms of IBS are resulting from interaction between environmental factors and factors related to genetically susceptible host (10). Induction of mucosal inflammation (caused by infections or any other unknown reason) results in increased permeability of mucosal barrier along small bowel and colon and subsequently activated secretory reflex and stimulated sensory roots among intestine. This group of patients present dominant symptoms of diarrhea (stimulation of secretory reflexes), abdominal pain (triggering of sensory roots) and bloating; induction of irritable bowel syndrome after infections, presence of mast cells and lymphocytes in intestinal mucosa, elevated level of inflammatory cytokines and clinical response to non-absorbable antibiotics are all signs of mucosal inflammation dominancy among these patients. These findings introduced new horizons in discovering novel and more specific therapeutic approaches to treat such patients (16).
Based on the obtained data, some studies investigated the effects of mesalazine as a well-known drug to treat inflammatory bowel disease that markedly reduces mucosal immune cells, especially mast cells, and significantly improves general well-being, and bismuth subcitrate with anti-diarrheal, anti-inflammatory and antibiotic properties in treatment of such patients (10, 14-17).
2. Objectives
The current study aimed to evaluate the efficacy of mesalazine and bismuth subcitrate on patients with symptoms of bloating and diarrheal dominant IBS unresponsive to routine therapeutic approaches.
3. Methods
The study was performed from summer 2013 up to spring 2014 on 40 consecutive patients with IBS referred to outpatient GI clinic of Ahvaz Imam hospital, Iran, as a referral center. Inclusion criteria included confirmation of IBS diagnosis by two gastroenterologists based on Rome III criteria, presence of bloating as dominant complaint of the patient and normal total colonoscopy with random biopsies. Exclusion criteria included any history of celiac disease, absence of improvement by routine therapeutic approaches for at least one year, pregnancy, breast feeding, history of abdominal surgery, opiate abuse, non-steroidal anti-inflammatory drug (NSAID) usage, long term antibiotic receiving, renal failure, sever chronic liver disease, sensitivity to salicylates and history of any morbid disorders. The socio-demographic level of participants was determined based on their monthly income and level of education. Before inclusion in the study, all of the participants were requested to sign an informed consent and also report their clinical manager any problems or side effects during the study period. They were also free to leave the study at any time based on their own desire. The study protocol was approved by Ahvaz Jundishapur ethics committee (ajums.REC.1393.39) and it was also registered in Iranian registry of clinical trials (IRCT2014080814190N4).
All of the study subjects were treated by mesalazine (2 g/day) and bismuth subcitrate (120 mg BID) for six months. All of the patients were evaluated for symptoms such as severity and duration of abdominal pain, number of bowel habits per day and improvement of bloating by filling a questionnaire in the beginning, third month and end of the study. They were given their medication based on monthly number of tablets for each month and were also observed not to miss any dosing of drugs. After collection, the data were analyzed by SPSS software ver. 15. Descriptive statistical method was used to measure average and standard deviation and normal variations determined by Kolmogorov-Smirnov test. The predictive value < 0.05 was considered meaningful.
4. Results
Overall, the study included 42 patients and 33 of them (78.6%) were female. The mean age of patients was 35.9 years (ranged 22 - 67 years) and the mean weight was 66.3 kg (ranged 42 - 100); 40% (2 males, 18 females) were single and from the viewpoint of socio-economical level, 32%, 44% and 24% had high, medium and poor monthly income, respectively. The mean number of patients' family members in their living place was 5 (ranged 2 - 9) persons; 96% of the subjects were nonsmokers and just two had a history of occasional alcohol consumption (Table 1). A couple of patients had glucose intolerance (FBS > 100), four had hypothyroidism (under treatment and euthyroid during study period) and four had past history of valvular heart disease such as mitral valve prolapse (MVP). In 20% of the patients, the family history for IBD was positive. Ten patients had history of bloody diarrhea and no one had the history of any significant liver diseases (except mild fatty liver disease among two patients).
| Characteristics | |
|---|---|
| Male/female | 9/32 |
| Average age, y | 35.9 (22 - 67) |
| Mean weight, kg | 66.3 (42 - 100) |
| Average family member | 5 (2 - 9) |
| Smoking (%) | 2 |
| Occasional alcohol consumption (%) | 4.7 |
The most common symptoms of participants included incomplete defecation and tenesmus (41 patients, 97.6%), bloating (39 patients, 92.8%), abdominal fullness (35 patients, 83.3%), mucus discharge (30 patients, 71.4%), constipation (30 patients, 71.4%), lower abdominal pain (28 patients, 66.6%), anorexia (22 patients, 52%), sleep disorders (18 patients, 42.8%), diarrhea (16 patients, 38%), weight loss (9 patients, 21.4%) and fever (3 patients, 7%). After an average of six months of treatment with mesalazine one gram plus bismuth subcitrate 120 mg BID (3 - 11 months), 69.1% of patients reported improvement in symptoms more than 50% (16 patients, 38.1%), ranged 75% - 100%, and 13 patients (31%), ranged 50% - 75%, indicated overall symptoms relief. The most significant improvement was reported for bloating (85%). There were no major side effects and only 26% of patients complained of minor degrees of diarrhea.
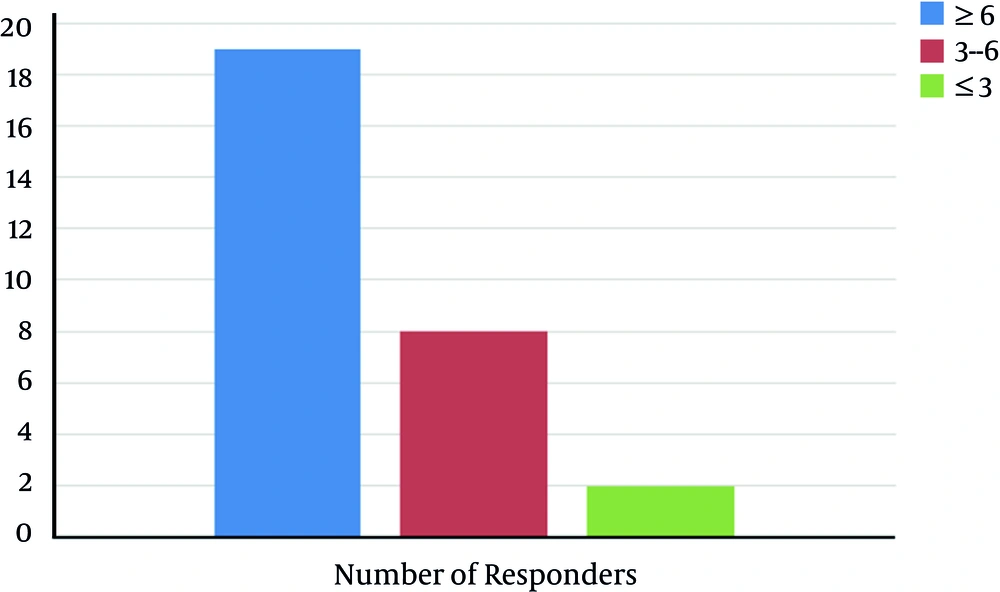
The rate of symptom improvement had no significant difference between males and females (P = 0.526). Moreover, the rate of > 50% improvement was unrelated to complaint or history of diarrhea (P = 0.33) and/or constipation (P = 0.526). The best therapeutic results were observed with treatment period of ≥ 6 months (Figure 1).
5. Discussion
Irritable bowel syndrome (IBS) is a common disease in most of the communities. In such settings, the patients complain of not only abdominal pain but also other gastrointestinal symptoms including bloating and eructation even though their presence is not mandatory for diagnosis (1-4). The current study evaluated the therapeutic efficacy of mesalazine and bismuth subcitrate regimen to treat patients with IBS and dominant complaint of bloating and incomplete defecation according to obvious effects of bloating on declining the quality of life of such patients and recent findings about the role of mucosal inflammation and immune system activation in pathogenesis of this condition (10-15).
The definite pathogenesis of this condition has not elucidated completely but disturbance of gut microbiota is considered among recent studies (12). Moreover, some studies showed that short course of antibiotic therapy resulted in significant improvement in GI symptoms of such patients (6). In a double blind clinical trial, neomycin resulted in 25% improvement among the results of lactulose breath test in comparison with that of placebo, and this drug was more effective in relieving clinical symptoms, although its usage was abandoned due to systemic absorption and side effects (18-20). Accordingly, in another study rifaximin, a non-absorbable antibiotic was more efficacious than placebo to treat bloating; although only 60% of participants in this study fulfilled the Rome II criteria to diagnose IBS and no one suffered through lactulose breath test (13). In the current study, prescribing bismuth subcitrate 120 mg plus mesalazine one gram BID resulted in significant improvement of bloating dominant IBS patients and this improvement remained up to two weeks after discontinuation of the drug. Some studies reported that this efficacy was more permanent; therefore, it seems that these effects could not be related to the therapeutic efficacy of bismuth subcitrate. One study evaluated efficacy of a three-week course of treatment with bismuth subcitrate and achieved a significant improvement among abdominal pain, changing bowel habit, diarrhea and also positive histologic changes in intestinal biopsy (15).
On the other hand, some studies considered mesalazine efficacious in decreasing mucosal lymphocytes and mast cells and general wellbeing among patients with IBS, even though not effective in relieving abdominal pain and bloating (14, 16, 17).
Based on the study findings, it was concluded that a combination of bismuth subcitrate as a non-absorbable antibiotic with mesalazine as an anti-inflammatory drug can be useful to treat bloating dominant in patients and as mentioned previously, no study evaluated this combination formula. Although the study was performed as a case series, since almost all of the subjects were chronically affected and treated by multiple different therapeutic regimens (including placebo and reassurance), authors could compare the current results of the participants with those of themselves as a control group; and may be in future the group can be categorized as a distinct group located in the boundary between IBS and IBD as a spectrum (21). It seems that the patients with chief complaint of bloating and incomplete defecation who did not match completely with the criteria of IBS or IBD could be nominated as irritable colitis and they respond very well to a limited course of masalazine plus bismuth.
5.1. Conclusion
The results of the study were indicative of improvement and symptom relief in the majority of patients and it seems that treatment prolongation up to six months could be a key factor to achieve better clinical responses. It is recommend to evaluate this therapeutic regimen in further randomized clinical trials.