1. Context
Air pollution encompasses gaseous pollutants and particles. In another point of view air pollution can be classified into anthropogenic and biogenic. Anthropogenic air pollution is considered to be a serious public health problem and can be harmful to human health and welfare (1). Approximately 80% of people 65 years and over have one or more chronic diseases and about 50% of this group have activity limitations (2). Chronic obstructive pulmonary disease (COPD), also known as chronic obstructive lung disease (COLD), and chronic obstructive airway disease (COAD), among others, is a type of obstructive lung disease characterized by chronically poor airflow. It typically worsens over time. The main symptoms include shortness of breath, cough, and sputum production (3-5). Most people with chronic bronchitis have COPD (6, 7). Tobacco smoking is the most common cause of COPD, with a number of other factors, such as air pollution and genetics playing a smaller role (8). In the developing world, one of the common sources of air pollution is from poorly vented stoves used for cooking and heating. Long-term exposure to these irritants causes an inflammatory response in the lungs resulting in narrowing of the small airways and the breakdown of lung tissue known as emphysema (3, 9). Advanced COPD leads to corpulmonale (high pressure on the lung arteries, which strains the right ventricle of the heart) and leads to symptoms of leg swelling and bulging neck veins (9-11). Chronic obstructive pulmonary disease is more common than any other lung disease as a cause of corpulmonale (10). It often occurs along with a number of other conditions, due in part to share risk factors (8). These conditions include: ischemic heart disease, high blood pressure, diabetes mellitus, muscle wasting, osteoporosis, lung cancer, anxiety disorder and depression (8). In those with a severe disease, a feeling of always being tired is common (7). Fingernail clubbing is not specific to COPD and should prompt investigations for an underlying lung cancer (12). Chronic obstructive pulmonary disease can be prevented by reducing exposure to the known causes (9). An acute exacerbation (a sudden worsening of symptoms) is commonly triggered by infection or environmental pollutants, or sometimes by other factors, such as improper use of medications (13, 14). Infections appear to be the cause of 50 to 75% of the cases, with bacteria in 25%, viruses in 25%, and both in 25%. Environmental pollutants include both poor indoor and outdoor air quality (14-16). Exposure to personal smoke and secondhand smoke increases the risk (14, 17). Cold temperature may also play a role, with exacerbations occurring more commonly in winter (18). Both indoor and outdoor air quality can be improved, which may prevent COPD or slow the worsening of existing disease (17). This may be achieved by public policy efforts, cultural changes, and personal involvement (9). A number of developed countries have successfully improved outdoor quality through regulations. This has resulted in improvements of the lung function of their populations (17). Those with COPD may experience fewer symptoms if they stay indoors on days when outdoor air quality is poor (9). Results from the worldwide studies showed that COPD affects 329 million people or nearly 5% of the population. In 2013, it ranked as the fourth-leading cause of death, killing over 3 million people (19). The number of deaths is projected to increase due to higher smoking rates and an aging population in many countries (20). It resulted in an estimated economic cost of $2.1 trillion in 2013 (21). Results showed that a significant increase in hospital admission for COPD, cardiovascular disease, ischemic heart disease and myocardial infarction was attributed to the increase in the nitrogen dioxide (NO2) concentration (22). In another study which was conducted in Taiwan, there was an association between the NO2 levels and in hospital admission in patients suffered from ischemic stroke, COPD and asthma exacerbation (23). Another study has shown associations between hospital admissions for cardiovascular diseases and CODP attributed to an increase in the NO2 concentrations (24). Dockery et al. in a cohort study has shown an adverse health impact of long-term air pollution exposure in the six U.S. cities. This study demonstrated that chronic exposure to air pollutants is independently related to cardiovascular mortality (25). In similar work, Mohammadi et al. studied the association between COPD and NO2 levels in the Ahvaz in 2009 (26). Also, Goudarzi et al. studied the association between COPD and NO2 levels in the Tehran in 2009 (27). Zalaghi et al. studied the association between COPD and NO2 levels in the Ahvaz, Bushehr and Kermanshah in 2010 (28).
From past to now, Ahvaz has been well-known due to industries as well as environmental pollution. In the last decade, an anthropogenic source of air pollution (dust storm) has joined to other environmental problems (29). Physical, chemical and biological characteristics of dust storm and also the identification of hazardous air pollutants, such as BTEX have been well-documented (29-32). The social impact of dust storm on Ahvaz citizens was also evaluated (33). Furthermore, health effects of air pollution attributed to NO2, ozone and particulate matter were reported in most megacities of Iran, particularly Ahvaz. It should be noted that ahvaz has suffered from dust storm recently (38).Therefore, we decided to assess health effects of NO2 which has not studied yet (1, 29, 33, 34).
The purpose of this study was to assess the COPD attributed to the NO2 exposure on human health in Ahvaz city (located in south-western Iran) during 2011-2012.
2. Evidence Acquisition
In this epidemiological study, sampling and data collection were done by Ahvaz Department of Environment. Nitrogen dioxide data were analyzed using the Excel software and AirQ model. The AirQ software was proved to be a valid and reliable tool to estimate the potential short-term effects of air pollution, predicts health endpoints attributed to criteria pollutants, and allows the examination of various scenarios in which emission rates of pollutants are varied (33). This study was conducted to assess the potential effects of nitrogen dioxide exposure on human health. Chronic obstructive pulmonary disease attributed to nitrogen dioxide in Ahvaz city at 2011 was calculated based on the utilizing relative risk (RR) and attributable proportion. Geographical features of Ahvaz: Ahvaz city, with a population of 1 million approximately, with an area of 8152 km2,the capital city of Khuzestan Province is located between 48 degree to 49°29′ east of Greenwich meridian and between 31 degrees and 45 minutes to the north of the equator (1). Sampling was performed for 24 hours in 4 stations. In this study 4×365 samples of Ahvaz’s air was taken and collected. Data was taken from Ahvaz Department of Environment (ADoE). Stations were Downtown “Naderi”, Old School of Public Health “Behdasht Ghadim”, Bureau of Meteorology “Havashenasi” and Head office of ADoE “Mohitzist”. To make the file, the following steps were taken in a row: these data were in volumetric base. Health effects are being related to the mass of pollutants inhaled and this is why the AirQ model was on gravimetric basis. Therefore, there was an inconvenience between AirQ model and ADoE data. Conversion between volumetric and gravimetric units include: correction of temperature and pressure (when the temperature and pressure change the volume of the gas changes, but it still contains the same mass of material), conformity of the unit with the model, coding, processing (averaging) and filtering are implemented for solving such problem. The daily mean was calculated based on codification, condition modification and primary and secondary filtering. Thereafter, 24 hour means were calculated for nitrogen dioxide pollutant. To estimate the health impact attributed to the exposure of air pollution on the target population, the AirQ model was used which estimatesthis impactfor specific air pollutants on a resident population in a certain area and period.
2.1. Data Analysis
We determine the extent of health effect based on the relative risk and attributable proportion attributed to nitrogen dioxide. The RR is a measure of association between a disease or condition and a factor under study. It is calculated by dividing the incidence rate among those exposed to the factor by the incidence rate among those not exposed to the factor (35).
RR = Incidence in the exposed population / Incidence in the non-exposed population
The population prevented fraction refers to situations where exposure to a factor is protective. Data capture was collected for criteria air pollutants. Attributable proportion is a fraction of health consequences in a specific population that can be attributed to a specific air pollutant exposure with this notion that there is a proven causative correlation between health consequences and air pollutant exposure. We have use default model that attribute the COPD cases to exposure to nitrogen dioxide and remove another factor effects. In addition to total cases attributable to exposure to nitrogen dioxide, we could estimate the distribution of cases attributable in terms of concentration intervals of pollutant. Having relative risk in a specific concentration level of the pollutant and rate in an unexposed population, we can obtain extras rate (I + (c)) and the number of extras (N + (c)) in a groups exposed (36):
(RR(c-1)) = I + (c) × INe P(c)
All the above-mentioned equations are based on the assumption that the estimation used in this analysis has been controlled regarding all probable confounders. Putting confidence intervals of the RR estimation in the equation, we will have upper and lower limits of attributed part and the range of cases attributable to expected exposure. Indeed, practically uncertainty of the effect (and the range of estimated effects) is larger due to exposure assessment errors and non-statistical uncertainties of concentration-response function. Thus, we may obtain the increase in death toll due to increase in the concentration of pollutants. Sampling and data collectionwere done atADoE and the data were analyzed using the Excel software and AirQ model. The health impact attributable to the exposure of air pollution on the target population was estimated using the AirQ model, which estimates this impactfor specific air pollutants on a resident population in a certain area and period (Figure 1).
Location of the Study Area and Sampling Station in the Khuzestan Province (Ahvaz City), in the South West of Iran (37)
3. Results
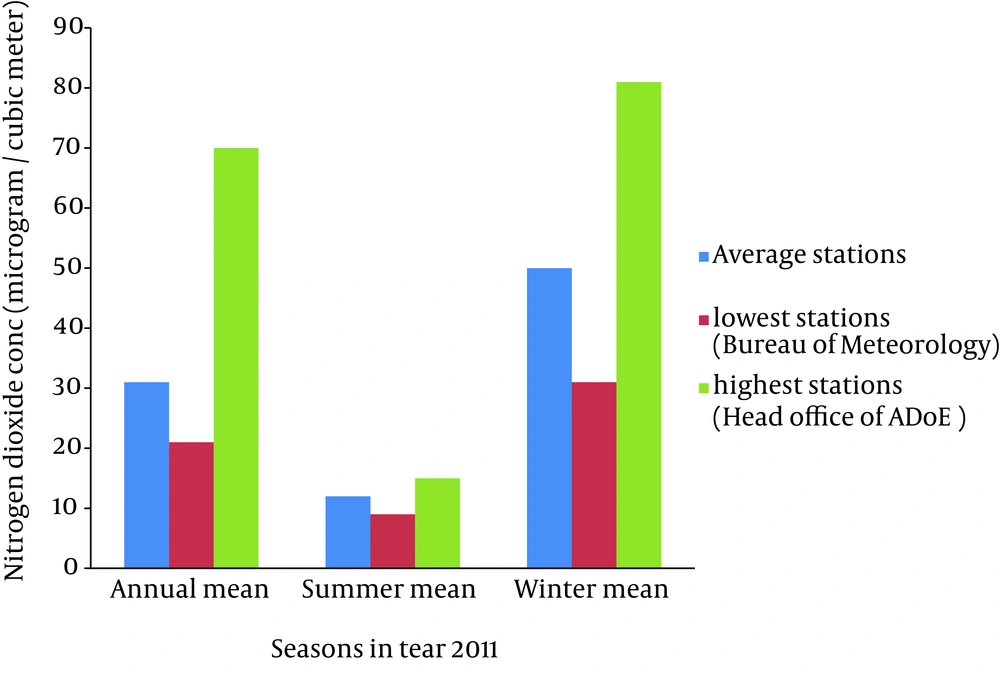
According to the research findings, the highest and the lowest annual average nitrogen dioxide concentrations during 2011-2012 were 70 and 21 μg/m3, respectively. The Bureau of Meteorology “Havashenasi” and Head office of ADoE “Mohitzist” had the highest and the lowest nitrogen dioxide concentrations during 2011-2012, respectively. The primary standard of nitrogen dioxide according to National Ambient Air Quality Standard (NAAQS) is 100 ppb as hourly averaging time (39). World Health Organization (AQG WHO) has recommended 40 and 200 μg/m3 as annual and hourly averages of nitrogen dioxide concentrations, respectively (34, 39). Table 1 shows that annual average of nitrogen dioxide concentration in Ahvaz was 31 μg/m3 in 2011, which is lower than AQG WHO and also much lower than the NAAQS standards. The annual average, summer average, winter average and 98 percentile of nitrogen dioxide concentrations in these stations have been presented in Table 1. According to Figure 2 in winter nitrogen dioxide concentrations were the maximum concentration as compared to other seasons during this year. This can result from pollutants produced in combustion processes which can occur mostly in transportation, power stations, heating plants and industrial processes. The major sources of nitrogen dioxide that can increase the risk of developing COPD are resulting from anthropogenic source of air pollution such as road traffic, stationary combustion and industrial processes. Ahvaz has been well-known due to its industries as well as petroleum steel and power stations. In the last decade, an anthropogenic source of air pollution has joined to other environmental problems.
| Stations/Parameter | Average Ahvaz | Lowest Stations (Mohitzist) | Highest Stations (Havashenasi) |
|---|---|---|---|
| Annual mean | 31 | 21 | 70 |
| Summer mean | 12 | 9 | 15 |
| Winter mean | 50 | 31 | 81 |
| 98 percentile | 148 | 61 | 286 |
The Highest and Lowest Concentrations of Nitrogen Dioxide (μg/m3) Corresponding to Stations
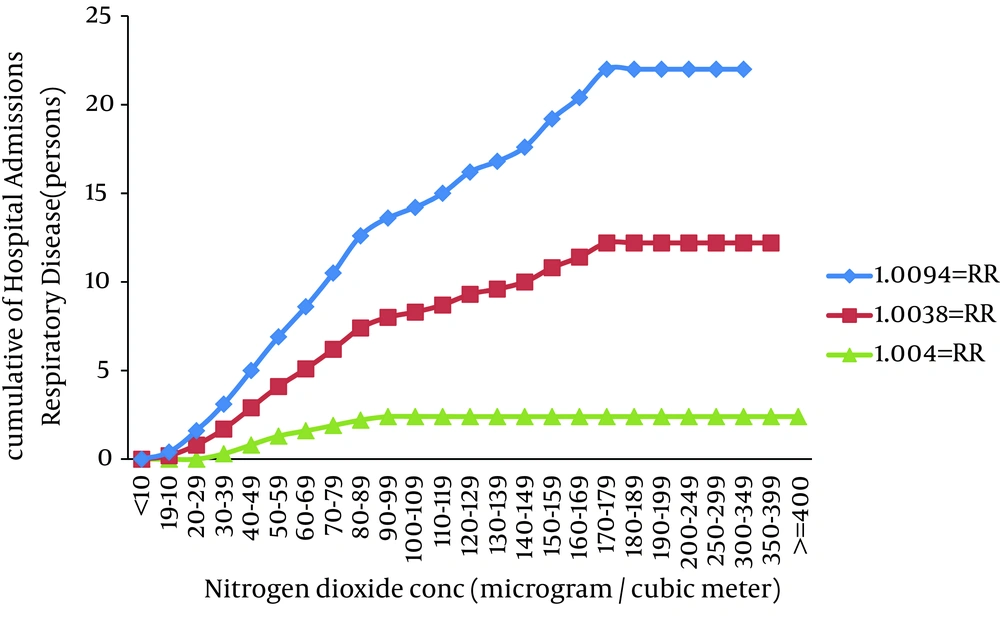
In field of morbidity, hospital admission of COPD versus nitrogen dioxide concentration has shown in Table 2. Estimated number of excess cases attributed to nitrogen dioxide for hospital admission of COPD at lower, central and higher confidence interval of RR was 3, 10 and 23, respectively. The lower level of RR may imply to the improvement of urban air quality by implementing of emission control strategies. Therefore, the higher RR can depict mismanagement in urban air quality. Central RR is corresponded to 10 as predicted number of excess cases and it can be a good representative of real situation in standpoint of nitrogen dioxide health effects. As Figure 3 indicates, despite the relative risk of health effects of nitrogen dioxide concentrations below 30 μg/m3 due to lack of contact with the population concentration is zero. In other words, no one day in 2011 has been reaches the nitrogen dioxide concentration below 30 μg/m3. Rising trend of cases hospital admission of COPD with increasing concentrations of Nitrogen dioxide in 30-170 μg/m3 has a uniform trend.
| Indicator/Estimate | RR (Medium) | AP, % | Attributable Excess Cases (Persons) |
|---|---|---|---|
| Down | 1.0004 | 0.092 | 2.4 |
| Mediocre | 1.0038 | 0.9561 | 9.8 |
| Up | 1.0094 | 1.1694 | 22.5 |
Relative Risks, Attributable Proportions and Number of People Suffering From Chronic Obstructive Pulmonary Disease Due to Nitrogen Dioxide Exposure
4. Conclusions
In recent decades, air pollution is considered as a serious threat to the environment, quality of life and health of people. This study aimed to estimate the effects of exposure to nitrogen dioxide air pollution on the risk ofdevelopingCOPD using the AirQ model in Ahvaz, Iran. Figures 2 to 3 have illustrated nitrogen dioxide concentrations versus related health endpoint and average concentrations during seasons. As the results showed, three ranges of RR based on model's default were considered for assessing health effects of nitrogen dioxide. Furthermore, BI values were also taken from default of the model. Also, results showed that winter and summer had the highest and the lowest nitrogen dioxide concentrations during 2011-2012, respectively. In Ahvaz population study on one million people and base on BI of 497 per 100,000 people in year 2011, 73% of the COPD cases occurred in days with pollutant not exceeding 70 μg/m3.
In similar work Goudarzi et al. they exploited the AirQ model to estimate the nitrogen dioxide hygienic effects on potential COPD in Tehran (capital of Iran). Based on their results, almost 4.4% of all cases of whole COPD are attributed to the nitrogen dioxide concentrations greater than 30 μg/m3 (28). Moreover, Mohammadi et al. report that approximately 3 percent of hospital admission for COPD happened when the nitrogen dioxide concentration was over 20 μg/m3(26).
The mortality rate due to COPD in Wisconsin was reported by 88% between 1980 and 2000 (40). Zalaghi et al. In 2010 in survey of health effects of air pollution in Ahvaz, Bushehr and Kermanshah report that approximately 3.5 percent in Ahvaz, 2.1 percent in Kermanshah and 1.1 percent of COPD attributed to nitrogen dioxide in people (28). The increase in the developing world between 1970 and the 2000s is believed to be related to increasing rates of smoking in this region, an increasing population and an aging population due to less death from other causes, such as infectious diseases (8). The global numbers are expected to continue increasing as risk factors remain common and the population continues to get older (41). Between 1990 and 2010 the number of deaths from COPD has decreased slightly from 3.1 million to 2.9 million (42). Overall, it is the fourth-leading cause of death (8). In some countries, mortality rate has decreased in men but has increased in women (43). This is most likely due to rates of smoking in women and men becoming more similar COPD is more common in older people; it affects 34-200 out of 1000 people older than 65 years, depending on the population looked at (3, 6, 44). In England, an estimated 0.84 million people (of 50 million) have a diagnosis of COPD; translating into approximately one person in 59 receiving a diagnosis of COPD at some point in their lives. In the most socioeconomically deprived parts of the country, one in 32 people were diagnosed with COPD, compared with one in 98 in the most affluent areas (45). In the United States approximately 6.3% of the adult population, totaling approximately 15 million people, has been diagnosed with COPD (46). 25 million people may have COPD if currently undiagnosed cases are included (47). In 2011, there were approximately 730,000 hospitalizations in the United States for COPD (48). The major limitations of this study is lack of databases and indicators amounts that for solve this lack we used the values of the WHO (Middle East) for calculated health effects attribute nitrogen dioxide. Therefore, estimating the health effects of air pollutants actually requires epidemiologic studies for accurate calculation of RRs and BIs. Accordingly, cost-effective measures and management schemes should be considered to abate air pollution concentrations and/or reduce the exposure of general population to air pollutants. Finally, the survey results showed that implementation of basic actions to the control of NO2 entering into the stratosphere by decreasing consume gasoil fuel in industries and development of green space is essential.
Based on the results of this study, 4% of hospital admissions for COPD were attributed to respiratory nitrogen dioxide in people. High percentage of the observed health endpoints was associated with high concentration of measured nitrogen dioxide. Although the results of this study are in line with results of other researches around the world, the geographic, demographic, and climate characteristics are different, there is still high need to further studies to specify local RR and BI. Using alternative energy sources, such as solar cooking and electrical heating is effective, as is using fuels such as kerosene or coal rather than biomass. Considering that little prerequisite building, calculated times diseases attributable to air pollution and health effects of air pollutants estimated, there are the epidemiological indicators, these indicators to calculate the experts are recommended.