1. Background
Obesity is not only associated with chronic diseases, such as cardiovascular disease, diabetes, hypertension, hyperlipidemia, and cancer, but is also a cause of mortality (1). The results of previous studies in Iran suggest that the obesity epidemic is growing rapidly and uncontrollably among children, youth, adolescents, and adults. Physicians and health professionals in Iran consider obesity and overweight as risk factors for cardiovascular diseases and diabetes and underline the irreversible consequences of these conditions (1, 2).
One of the main objectives of the international diabetes day is to fight and prevent obesity. Apart from genetics, the main reason for the high prevalence of obesity is the energy imbalance in the body, which results in an increase in body fat. Various factors such as harmonic and metabolic abnormalities contribute to obesity. In addition, obesity and cardiorespiratory fitness (CRF) are risk factors for cardiovascular mortality. On the other hand, maximal oxygen consumption (VO2max) is considered the gold standard for CRF. Reduced CRF or VO2max is associated with systemic inflammation (3). In the literature, the negative correlation between obesity and VO2max suggests the effect of excessive body fat on cardiorespiratory functions (3).
Recently, the role of inflammatory cytokines, such as interleukin-6 (IL-6), IL-10, IL-1β, and tumor necrosis factor-α (TNF-α), has been widely reported in patients with obesity, overweight, and related abnormalities (2). Meanwhile, IL-1β is a well-known cytokine among regulating proteins of cell physiological responses, known to trigger insulin resistance and cause or increase the intensity of some obesity-related diseases due to its inflammatory features (4).
IL-1β is a proinflammatory cytokine and a member of IL-1 family of cytokines. This cytokine is produced as a proprotein by activated macrophages and participates in the regulation of immune responses, inflammatory reactions, and hematopoiesis (5). Besides inflammatory and respiratory diseases, inflammatory cytokines are also related to metabolic abnormalities, affecting obesity and body fat. Therefore, an increase in systematic levels of cytokines causes an increase in insulin resistance and glucose homeostasis disorders (6).
The level of IL-1β increases in the presence of obesity (7); in fact, some studies directly link IL-1β level to body mass index (BMI) (8). On the other hand, it plays a major role in fat metabolism through regulating insulin level and lipase activity (9). Therefore, reduction in its level due to internal or external interventions, such as diet or exercise training programs, paves the way for reducing insulin resistance, as well as the intensity of type II diabetes or other fat-related diseases (10). Some studies have associated the improvement of inflammatory profile to an increase in CRF or weight loss due to exercise training (11). However, exercise training or other external stimuli have been reported to have different effects on the level of this cytokine or other inflammatory mediators in obese people or those with related diseases.
In this regard, Balducci et al. (2009) indicated that long-term, low-intensity exercise training and aerobic-resistance hybrid programs decreased IL-1ß level in obese and diabetic people (12). However, Lopes et al. (2016) found no significant changes in other inflammatory markers, such as adiponectin, insulin resistance, IL-6, TNF-α, and IL-10 in response to 12 weeks of aerobic training in overweight girls, despite a decline in leptin or C-reactive protein (CRP) (13). In another study, six months of a weight-loss program in form of diet and exercise training yielded no changes in IL-1β level among obese women (14). On the other hand, in another syudy (2008) observed a significant increase in IL-1β level among laboratory rats following 8 weeks of regular aerobic training (15).
Some studies on changes in serum IL-1β level in different exercise training programs reported no changes after 8 weeks of resistance training in healthy men (16). On the other hand, a significant decline in serum IL-1β level was reported after 6 weeks of moderate- and high-intensity resistance training in healthy men (17). Moreover, a significant reduction was found after moderate- and high-intensity aerobic training in inactive obese men (18). A review of the mentioned studies indicated inconsistent and controversial results regarding changes in IL-1β and other inflammatory mediators due to exercise training, which makes it difficult to draw a general conclusion and calls for further research. Moreover, few studies have been performed among obese women.
2. Objectives
The present study aimed to determine the impact of an endurance training program on IL-1β level as one of the most important inflammatory markers, as well as VO2max as the most important index for the evaluation of CRF in obese women.
3. Methods
3.1. Subjects and Inclusion Criteria
In this semi-experimental study, 30 sedentary obese women (30 ≤ BMI ≤ 36), aged 30 - 40 years, were selected through convenience and purposive sampling. They were then divided into exercise (3 months of endurance training; n, 15) and control (no training; n, 15) groups, based on random sampling using a table of random numbers (Fall 2016, Saveh, Iran). The experimental protocol was approved by the ethics committee of Islamic Azad University, Iran. All the participants provided written informed consents prior to randomization.
All the subjects were nonathletic and nonsmokers. The participants were included in the study if they had not been involved in regular physical activities in the past 6 months. None of the subjects used drugs or therapies for obesity, and none had a history of diseases or injuries, which would prevent daily exercise. On the other hand, pregnancy, planning for pregnancy, and history of eating disorders, were the exclusion criteria. Moreover, women with a diagnosis of coronary cardiac disease, chronic airway disease, impaired hepatic dysfunction, diabetes, acute diseases, or symptoms indicative of ischemia on electrocardiography were excluded.
3.2. Anthropometric Measurements
Height and weight of the participants were measured via standard procedures (barefoot, wearing underwear) with a Seca 220 scale (22089 Hamburg, Germany). The BMI was calculated as weight in kilograms, divided by the square of height in meters. Visceral fat and body fat percentage were determined using a body composition monitor (OMRON, Finland) (19). Abdominal circumference and hip circumference were measured in the most condensed part using a nonelastic cloth meter. Two measurements were done every 1 minute, and the average of these measurements was used for the analysis.
3.3. Training Protocol and Laboratory Assays
The endurance training program continued for 12 weeks (3 sessions per week), consisting of warm-up (10 minutes), main exercise, and cooling down (5 - 10 minutes) sessions. The main exercise took 30 - 45 minutes each session at an intensity of 60% - 75% HRmax: first and second weeks, 60% - 65% of HRmax; third and fourth weeks, 65% - 70% of HRmax; and fifth and sixth weeks, 70% - 75% of HRmax. Following that, the subjects continued aerobic exercises for 6 weeks until the twelfth week at 70% - 75% of HRmax.
Heart rate in each session was controlled by polar telemetry. To determine the intensity of exercise, heart rate was measured by counting the heartbeats via polar telemetry. The control subjects were instructed to maintain their habitual activities. The participants were instructed to maintain their usual diet throughout the study. CRF (measured as VO2max) was determined using the one-mile Rockport fitness walking test (20).
A venous blood sample was collected from all the subjects after 12 hours of overnight fasting between 8 and 9 a.m. (pretraining). The subjects were asked to avoid any heavy physical activity for 48 hours before blood sampling. After the final training, the subjects rested for 48 hours, and then, fasting blood samples were collected, similar to the pretest (posttraining). After each blood sampling, serums were immediately separated and stored at -80°C until further assays. The serum was used to measure IL-1β, and ELISA assay (Biovendor Laboratorial kit, Biovendor Company, Austria) was applied for the quantitative detection of human serum IL-1β. The intra- and interassay coefficients of variation were 5.1 and 8.6%, respectively.
3.4. Data Analysis
Normal distribution of data was analyzed by Kolmogorov-Smirnov test. Comparisons between parametric variables were performed by paired and unpaired sample t test. To determine the effect of aerobic training on various parameters (IL-1β, VO2max, and anthropometric markers), pre- and posttraining delta values were compared by independent t test for each variable. Paired t test was used to determine the mean differences between pre- and posttraining values for all the variables (intragroup comparisons). P value less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 15.0 (SPSS Inc., IL, USA).
4. Results
Table 1 presents the descriptive anthropometric and biochemical characteristics of the groups. All values are reported as mean and standard deviation. The results of independent t test showed no significant differences between the groups with regard to anthropometric markers at baseline (P > 0.05). In addition, no baseline differences were found between the groups for serum IL-1β and Vo2max (Table 1).
| Variables | Exercise Group | Control Group | Sig. |
|---|---|---|---|
| Age, y | 38.4 (2.47) | 38.9 (1.96) | 0.57 |
| Height, cm | 161.1 (4.85) | 161.3 (3.47) | 0.93 |
| Weight, kg | 83.3 (6.87) | 83.47 (5.40) | 0.94 |
| Abdominal circumference, cm | 112 (5.24) | 113 (3.97) | 0.61 |
| Body mass index, kg/m2 | 32.03 (1.26) | 32.07 (1.33) | 0.93 |
| Body fat, % | 45.04 (1.96) | 45.39 (1.64) | 0.60 |
| Visceral fat | 8.6 (0.91) | 8.47 (0.74) | 0.66 |
| IL-1β, pg/mL | 2.44 (0.57) | 2.35 (0.58) | 0.68 |
| VO2max, mL/kg/min | 34.51 (5.44) | 35.42 (4.18) | 0.53 |
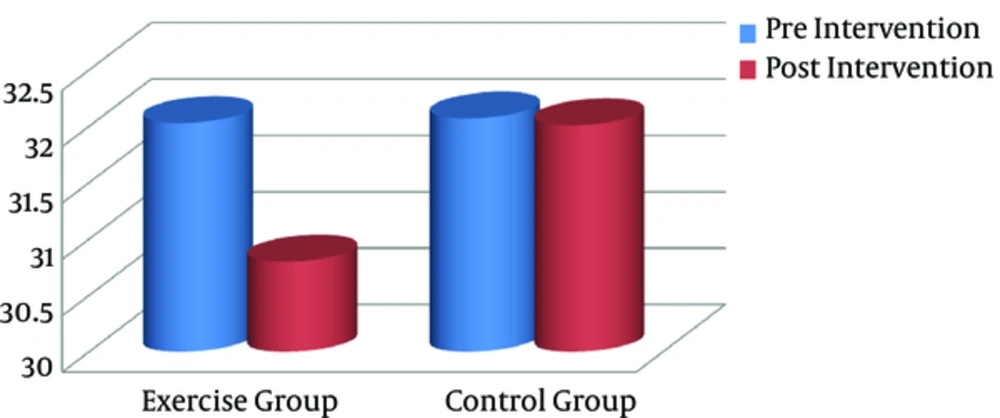
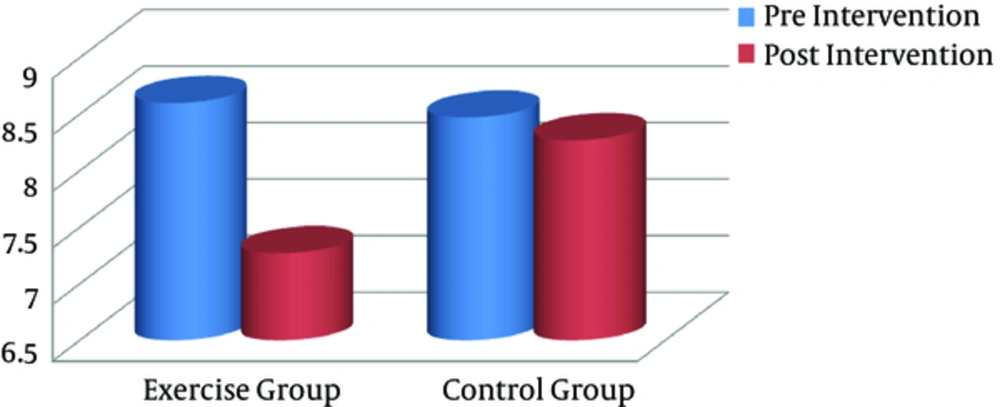
After the training program, all anthropometric markers were significantly lower in the exercise group, compared to the controls (P < 0.05). Paired sample t test was used to compare pre- and posttraining values (intragroup differences) in each group separately. Based on the finding, the exercise group showed a significant decline in BMI (Figure 1), visceral fat (Figure 2), and other anthropometric markers, compared to the baseline (Table 2). However, there were no significant changes in these variables in the control group.
| Variables | Exercise Group | Control Group | ||||
|---|---|---|---|---|---|---|
| Pretraining | Posttraining | Sig | Pretraining | Posttraining | Sig | |
| Weight, kg | 83.3 (6.87) | 80.08 (6.41) | 0.000 | 83.47 (5.40) | 83.31 (5.37) | 0.496 |
| AC, cm | 112 (5.24) | 106 (5.66) | 0.000 | 113 (3.97) | 113 (4.25) | 0.865 |
| BMI, kg/m2 | 32.03 (1.26) | 30.8 (1.19) | 0.000 | 32.07 (1.33) | 32.01 (1.30) | 0.498 |
| Body fat, % | 45.04 (1.96) | 40.66 (1.42) | 0.000 | 45.39 (1.64) | 45.08 (2.37) | 0.305 |
| Visceral fat | 8.6 (0.91) | 7.27 (0.45) | 0.000 | 8.47 (0.74) | 8.27 (0.88) | 0.189 |
Abbreviations: AC, Abdominal Circumference; BMI, Body Mass Index; HC, Hip Circumference.
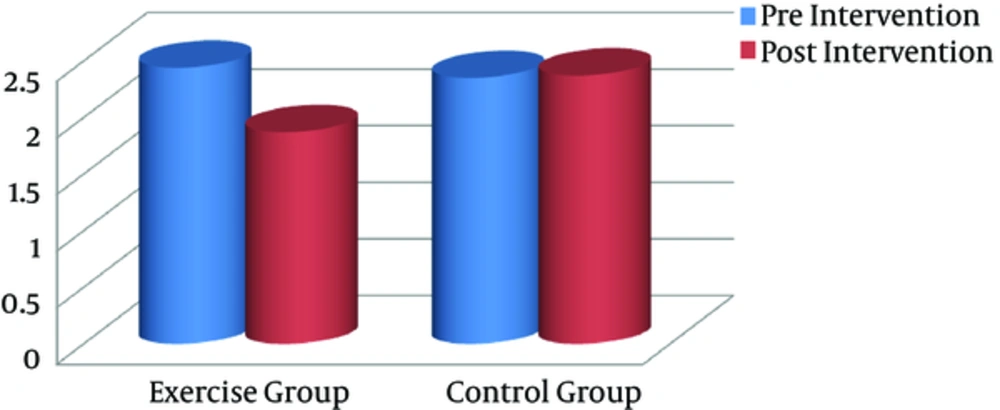
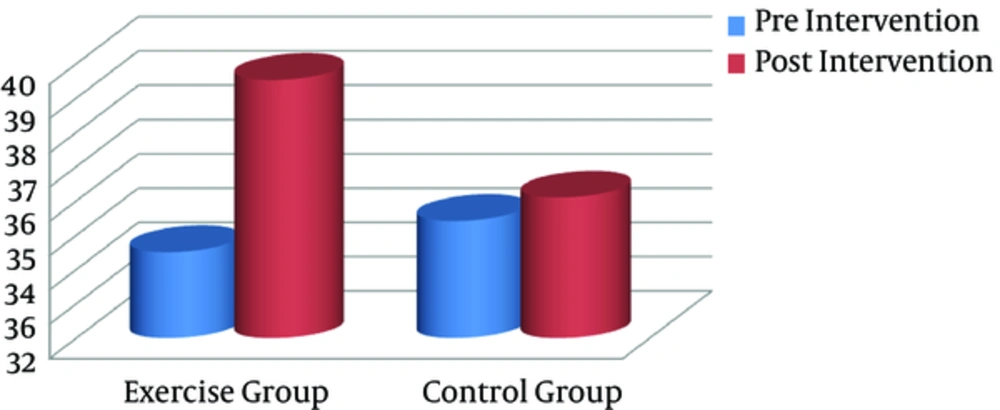
Significant differences were found between the groups with regard to serum IL-1β and VO2max after the training intervention (postintervention). In addition, intragroup changes showed that the endurance training intervention resulted in a significant decline in serum IL-1β level, compared with the baseline in the exercise group (P = 0.001), whereas it remained unchanged in the controls (Figure 3). Compared to pretraining, VO2max as a CRF index decreased significantly after the exercise program (P = 0.023), whereas it remained unchanged in the controls (Figure 4).
| Variables | Exercise Group | Control Group | ||||
|---|---|---|---|---|---|---|
| Pretraining | Posttraining | Sig | Pretraining | Posttraining | Sig | |
| IL-1β, pg/mL | 2.44 (0.57) | 1.87 (0.29) | 0.001 | 2.35 (0.58) | 2.37 (0.58) | 0.809 |
| VO2max, mL/kg/min | 34.51 (5.44) | 39.53 (7.36) | 0.023 | 35.42 (4.18) | 36.1 (6.56) | 0.543 |
5. Discussion
The significant reduction in serum IL-1β level following endurance training is one of the major findings of this study. In other words, a 3-month training intervention significantly reduced IL-1β level as one of the most important inflammatory markers in obese adult women. VO2max improvement as the CRF marker and reduction of anthropometric markers, including weight, body fat percentage, and visceral fat, are among the other findings of the current study. Although there have been few studies on IL-1β level, exercise training has shown diverse effects on other inflammatory mediators in the literature. Some of these studies are consistent, while some are inconsistent with the current study.
In a previous study, 12 weeks of endurance training with average intensity increased the serum IL-1β level in healthy people (20). In another study by Oh et al. (2013), six months of lifestyle changes in diet and exercise did not result in significant changes in IL-1β level or other inflammatory mediators, such as leptin, resistin, adiponectin, IL-6, and TNF-α in women with metabolic syndrome, despite significant changes in CRP (14). Azizbeigi et al. (2013) reported no significant changes in serum IL-1β level after 8 weeks of resistance training in healthy men (17).
In a study by Eisanezhad et al. (2012), eight weeks of aerobic training did not significantly change IL-1β or other inflammatory mediators, such as TNF-α and IL-6 in laboratory rats, despite a significant increase in the serum heat shock protein (Hsp70) (21). However, in a study by Martin et al. (2009), fourteen weeks of aerobic training caused a significant decline in IL-1β level in obese rats (22). In line with the current study, Balducci et al. (2009) observed significant changes in IL-1β level following long-term aerobic training in people with obesity and diabetes (12). In addition, in a study by Mehrabani et al. (2014), twelve weeks of aerobic training with moderate and high intensity resulted in a significant decline in serum IL-1β level among inactive obese men (18).
Long-term endurance training seems to be associated with antiinflammatory features in obese people through reducing IL-1β level. Clinical studies have noted the role of IL-1β as an inflammatory cytokine in healthy and unhealthy obese people, as well as patients without obesity (23). Some physiological and preinflammatory effects of IL-1β emerge directly or in combination with other inflammatory mediators. IL-1β is one of the primary CRP regulators and acute phase proteins, exuded by neutrophil granoliths and macrophages at damaged points (24). This inflammatory mediator imposes its biological responses by activating type I receptors of IL-1, namely, where IL-1 receptor antagonist (IL-1Ra) acts as an antagonist mediator (25).
High levels of this inflammatory mediator, especially in obese people, decrease insulin activity in cell membranes, increase insulin resistance, and cause or increase the intensity of type II diabetes through a secondary increase in lipocalin-2 and TNF-α (26). Therefore, a decrease in its level as a reaction to diet or regular exercise training results in the improvement of insulin resistance or reduces the intensity of diabetes, especially in obese people (10). Based on this finding, weight loss intervention is the principal nonpharmacological method for obese/overweight individuals to prevent or treat related diseases such as type II diabetes (27).
It is possible to relate the significant decrease in IL-1β level to weight loss or body fat tissue loss as a response to endurance training in this study. Several studies have supported the potential effects of weight loss and body fat tissue loss on the inflammatory profile following diet or exercise training. On the other hand, in a study by Donges et al. (2013), weight loss and abdominal fat loss caused an improvement in inflammatory mediators, such as IL-6 and TNF-α after 12 weeks of aerobic training in overweight men (28). The positive effects of weight loss on the inflammatory profile after long-term exercise training have been reported in other studies (29).
Bijeh et al. (2013) found that weight loss and body fat tissue loss caused no significant reduction in IL-1β level as a response to calorie restriction, along with exercise training after 27 sessions of aerobic training during Ramadan (30). Reduction in IL-1β level, through controlled diet and exercise training, enhanced insulin resistance in healthy and sick people (10). In this context, some previous studies have suggested that a minimum weight loss of 5% is required to improve the adipokine profile and several transcriptions of proinflammatory genes in obese people; these changes in adipocyte physiology may be associated with the reduced risk of metabolic diseases in this population (31, 32).
The balance or improvement of inflammatory profile as a reaction to exercise programs may be limited to the skeletal muscle level, not the systematic level. For instance, six months of exercise training (4 to 6 sets of 10-minute bicycling every day) decreased TNF-α, IL-6, and IL-1β levels in skeletal muscles (not systematic levels) among people with heart diseases (33). The increase in fat tissue due to advancing age or the increase in antiinflammatory cytokines from unicore blood cells resulted in the reduction of muscle tissues (34). Muscle contractions resulting from long-term aerobic or resistance exercise training decreased inflammatory muscle cytokines, while weight loss as a result of diet did not yield the same results (34).
Reduction in serum IL-1β level as a response to endurance interventions improved CRF in obese women, whereas exercise training did not significantly increase VO2max. Therefore, IL-1β improvement can be also related to an increase in VO2max as a result of aerobic training. In consistence with this finding, some studies have reported improvements in both VO2max and inflammatory profile (33). In this study, exercise training resulted in a 29% increase in the operational capacity of patients (33).
In a study by Brunelli et al. (2015), the improved inflammatory profile in response to 24 weeks of combined training was attributed to the increased VO2max in obese, middle-aged men (35). Based on these observations, researchers have related the improvement of these cytokines to an increase in CRF in reaction to training programs for patients. Other studies have also reported simultaneous improvements in CRF and inflammatory profile in response to exercise training (36). Furthermore, Karch et al. (2013) reported that an increase in VO2max in reaction to exercise training reduces the inflammatory profile (37). Babbitt et al. (2013) also found a significant relationship between relative VO2max and IL-10 changes following 6 months of aerobic training (10).
However, some other studies have found inflammatory cytokines to be independent of CRF (38); in other words, simultaneous improvements occurred in VO2max and some cytokines (not other inflammatory markers). For instance, Lopes et al. (2016) found no significant changes in adiponectin, resistin, IL-10, and TNF-α levels despite an improvement in VO2max, leptin, and CRP following 12 weeks of hybrid training (resistance aerobic training) in overweight girls (13).
Some researchers believe that exercise training decreases inflammatory cytokines at higher basic levels, independent of changes in weight or body fat percentage. This is indicative of the fact that basic inflammation levels are an important factor in response to exercise training (39). Irrespective of these observations, improvement in both CRF and IL-1β significantly changed body fat tissue and weight after 3 months of endurance training in obese adult women. The significant decline in IL-1β can be somehow construed as an adaptation to long-term training. Research on people with obesity and type II diabetes has shown that long-term exercise training decreases inflammatory signs by improving IL-1β level (40).
The strength of the present study is related to the improved level of IL-1β in response to endurance training without dietary changes. On the other hand, the low number of participants is one of the limitations of this study. In addition, responses of other inflammatory or antiinflammatory cytokines, such as IL-6, TNF-α, and adiponectin, were not measured, which is another shortcoming of this study.
5.1. Conclusion
Three months of endurance training significantly decreased IL-1β level as one of the most important inflammatory markers in inactive obese women. IL-1β reduction following endurance training produced obesity markers, such as abdominal obesity and body fat percentage loss, and increased CRF. Therefore, based on the recent findings and some former studies, it can be concluded that IL-1β reduction results from an increase in CRF and a decline in body fat tissue.