1. Background
The prevalence of gastric adenocarcinoma is rising worldwide. This disease is recognized as the fourth most prevalent cancer and the third cause of mortality worldwide (1). In developing countries, the cumulative risk has been estimated at 1.1%, and the associated mortality rate is as high as 0.9%. As 70% of all new cases and deaths of gastric adenocarcinoma are reported in developing countries (2), prevention and treatment are essential in countries such as Iran.
Complementary and alternative medicine (CAM) provides treatment options for gastric cancer. Based on a recent meta-analysis, CAM treatments have grown in popularity in many countries (3). One of the herbal treatments for gastric cancer complications in Iran is Spinal-Z. This medication is a methanolic mixture of dried seed powder of Peganum harmala Linn and leaf of Dracocephalum kotschyi Boiss. It is available as edible capsules and has been used to treat various cancers. According to the literature, this herbal medicine can reduce the viability of cancerous cell lines in mice (4).
Peganum harmala is one of the traditional treatments for various diseases including cancer. Based on previous reports, alkaloids found in this plant can prevent tumor cell growth. This plant is an herbaceous perennial plant of the family Zygophyllaceae, native to different regions, including countries of the Middle East such as Iran (4, 5). It can also improve cell lysis within 24 hours after use (6).
Traditionally, Dracocephalum kotschyi has been used independently as a treatment for rheumatic diseases due to its inhibitory effects on lectin-induced cellular immune responses. Moreover, another component of Spinal-Z, calycopterin, can inhibit mitogen-induced lymphocyte proliferation (7). Calycopterin can also induce anticancer effects due to the involvement of P-CREB and phase-II detoxifying enzyme system (8). Xanthomicrol is also one of the main cytotoxic components of Dracocephalum kotschyi and a potential anticancer agent (4).
Considering the molecular structure of Spinal-Z, this physiologically compatible agent has no major side effects (4). Moreover, it has cytotoxic, antiinflammatory, antiedema, pain relief, antibacterial, and antiviral effects (6). With this background in mind, the aim of the present study was to evaluate the effect of Spinal-Z on gastrointestinal symptoms of patients with gastric adenocarcinoma.
3. Methods
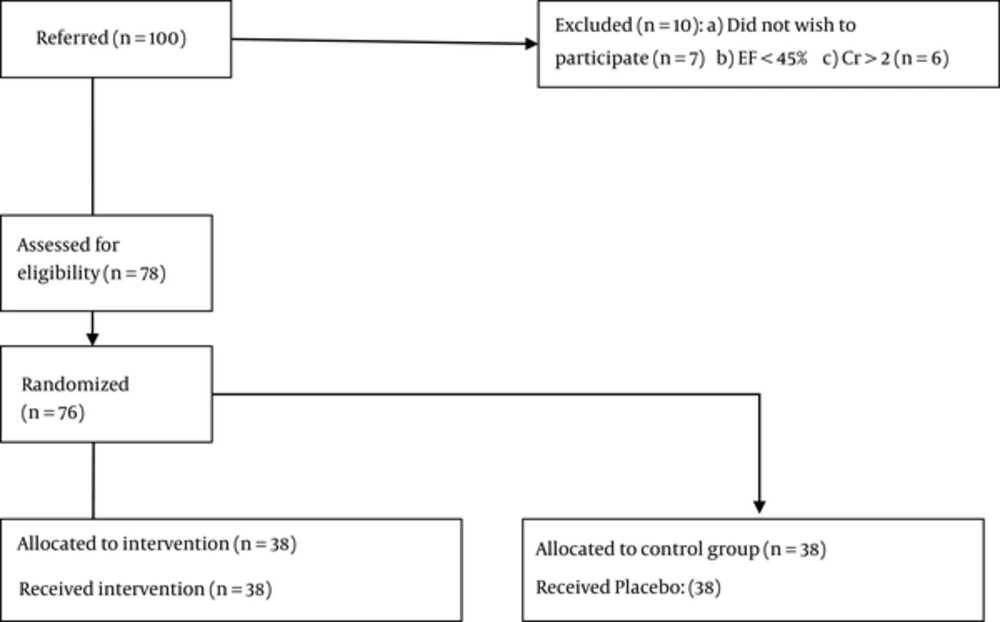
In this double-blind randomized controlled trial, 76 patients with gastric adenocarcinoma, admitted to Shafa Hospital (a teaching hospital), were allocated to the intervention and control groups. The inclusion criteria were as follows: 1) metastatic phase of cancer (stage IV); 2) no use of chemotherapy drugs during the study; and 3) diagnosis of gastric adenocarcinoma. On the other hand, the exclusion criteria were serum creatinine level > 2 mg/dL and ejection fraction < 45%.
A total of 76 patients were randomly assigned into the intervention and control groups (n, 36 per group). The intervention group received 6 Spinal-Z capsules (2 after breakfast, 2 after lunch, and 2 after dinner). The control group received placebo capsules in the same manner for 24 weeks.
3.1. Medications and Measurements
Spinal-Z was manufactured and formulated by Darupakhsh Co. (Iran) and contained 200 mg of Peganum harmala Linn. (20%) and Dracocephalum kotschyi Boiss (80%) powder. The medications were given to the participants free of charge every week, and patients’ adherence to the regimen was monitored via phone calls every day in both intervention and control groups.
The following parameters were measured using the participants’ blood samples: systolic blood pressure, diastolic blood pressure, heart rate, blood electrolytes including sodium, potassium, and creatinine, fasting blood sugar (FBS) level, erythrocyte sedimentation rate (ESR), liver function enzymes including aspartate aminotransferase (AST) and alanine aminotransferase (ALT), hemoglobin level, white blood cell (WBC) count, platelet count, alkaline phosphatase level, total bilirubin level, and direct bilirubin level. All the measurements were performed at baseline and after 2, 8, 12, 16, and 24 weeks.
3.2. Ethical Considerations
All institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research. Written informed consents were obtained from all the participants, and the ethics committee of Ahvaz Jundishapur University of Medical Sciences approved the study (Ref No., U-91175). The study protocol was registered at the Iranian registry of randomized controlled trials (IRCT2013010111967N1).
3.3. Statistical Analysis
The significance level was set at a 2-tailed type I error of 0.05. All statistical analyses were performed using SPSS version 22. Categorical data are reported as number (percentage), and continuous variables are presented as mean ± SD. Shapiro-Wilk test was used to examine the normal distribution of quantitative variables. To compare the demographic characteristics of the groups, independent sample t test or nonparametric Mann-Whitney U test (if needed) was used for continuous variables, and Pearson’s Chi square test was conducted for nominal variables.
Generalized estimating equation (GEE) (1) model was applied to examine the differences between the groups (intervention and control groups) in terms of changes in clinical measures (eg, serum creatinine level, FBS, blood pressure, heart rate, hemoglobin level, platelet count, WBC count, and liver enzymes) and side effects (eg, dysphagia, abdominal pain, anorexia, itching, nausea, and vomiting) over time. The GEE model included 2 main effects (group and time), as well as the interactions of these effects. Time points in the analyses included baseline and weeks 2, 4, 8, 12, 16, and 24. P value less than 0.05 was considered statistically significant, and all tests were 2-sided. SPSS version 18.0.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
4. Results
4.1. Participants
In total, 76 patients were randomized between December 2012 and January 2014. The patients were analyzed in terms of baseline characteristics (Figure 1).
4.2. Demographic Characteristics
The mean age of the participants was 56.16 years. Overall, 56.6% of the subjects were male (57.9% of the control group and 55.3% of the intervention group) and 43.4% were female (42.1% of the control group and 44.7% of the intervention group). The distribution of demographic and clinical characteristics was homogeneous in the intervention and control groups. The demographic characteristics of the participants are presented in Table 1.
| Variables | Intervention Group, n = 38 | Control Group, n = 38 | P Valueb |
|---|---|---|---|
| Age (years) | 57.45 ± 12.87 | 56.78 ± 11.6 | 0.81 |
| Gender | > 0.99 | ||
| Female | 17 (44.7) | 16 (42.1) | |
| Male | 21 (55.3) | 22 (57.9) |
aValues are expressed as Mean ± SD or N (%).
bThere was no significant difference between the groups.
4.3. Spinal-Z and Laboratory Parameters
The variables in the intervention and control groups were evaluated regarding the effect of time alone and interaction of time and treatment. Spinal-Z treatment had no significant effects on cardiovascular parameters, such as systolic blood pressure, diastolic blood pressure, or heart rate, compared to the control group; time also did not cause any changes in the outcomes. We did not detect any significant counteraction between time and treatment. Similar results were reported for the hematopoietic system (WBC count, platelet count, and hemoglobin level), hepatic factors (AST, ALT, alkaline phosphate, and total bilirubin levels), and blood electrolytes (sodium, potassium, and creatinine levels).
Spinal-Z could significantly decrease blood glucose level during the follow-up (P = 0.01); however, time-treatment interaction had no significance. We observed a significant decline in ESR in the intervention group during the follow-up (P < 0.05). In addition, a significant interaction between time and treatment was reported (P < 0.05).
4.4. Spinal-Z and Gastrointestinal Problems
Spinal-Z could significantly decrease gastrointestinal problems over time (P < 0.5).
4.5. Side Effects
Treatment with Spinal-Z did not have any effects on patients’ symptoms such as itching, nausea, and vomiting neither temporarily nor in the long run (after 24 weeks of follow-up; P > 0.05).
| Week 0 | Week 8 | Week 12 | Week 16 | Week 24 | P Valueb | ||
|---|---|---|---|---|---|---|---|
| Hemoglobin | Spinal-Z | 11.4 (1.07) | 11.4 (1.08) | 11.43 (0.94) | 11.8 (1.1) | 0.96 | |
| Placebo | 11.2 (1.1) | 11.1 (1) | 11.2 (0.88) | 11.2 (0.9) | |||
| WBC | Spinal-Z | 8080 (2210) | 8028 (1956) | 8014 (1770) | 8014 (1770) | 0.12 | |
| Placebo | 8220 (2200) | 800 (1800) | 7890 (1830) | 7770 (1670) | |||
| Platelet count | Spinal-Z | 171.47 (3800) | 181.18 (38.2) | 182.08 (93.2) | 180.74 (38.08) | 0.38 | |
| Placebo | 179.29 (37.3) | 179.13 (35.8) | 183.3 (39.8) | 179.7 (36.5) | |||
| Systolic blood pressure | Spinal-Z | 121.9 (14.1) | 122.7 (14.8) | 124.08 (19) | 122.7 (14.9) | 0.97 | |
| Placebo | 120.8 (18.8) | 120 (13.2) | 120.02 (15.1) | 119.6 (15.4) | |||
| Diastolic blood pressure | Spinal-Z | 78.6 (6.1) | 78.9 (6.4) | 79.6 (5.5) | 78.6 (4.1) | 0.79 | |
| Placebo | 78.1 (6) | 77.7 (5.5) | 75.9 (6.4) | 78 (4.8) | |||
| Heart rate | Spinal-Z | 78.3 (7.2) | 79.5 (6.1) | 79 (5.6) | 78.3 (4.8) | 0.32 | |
| Placebo | 80.6 (6.3) | 81.4 (5.5) | 80.5 (4.9) | 79.9 (4.5) | |||
| Creatinine | Spinal-Z | 1.1 (0.31) | 1.1 (0.3) | 1.11 (0.29) | 0.42 | ||
| Placebo | 1.1 (0.29) | 1.19 (0.28) | 1.19 (0.29) | ||||
| Calcium | Spinal-Z | 139.6 (2.2) | 139.3 (1.6) | 137.03 (1.6) | 0.41 | ||
| Placebo | 140 (2.09) | 139.89 (2.9) | 139.6 (2.03) | ||||
| Potassium | Spinal-Z | 4.08 (0.16) | 4.05 (0.15) | 4.05 (0.09) | 0.051 | ||
| Placebo | 4.05 (0.14) | 4.01 (0.08) | 4.09 (0.08) | ||||
| ESR | Spinal-Z | 59.08 (20.7) | 53.9 (16.6) | 40.7 (12.3) | < 0.05 | ||
| Placebo | 65.16 (21.9) | 63.18 (19.9) | 62.89 (20.9) | ||||
| FBS | Spinal-Z | 102.9 (20.7) | 100.3 (15.6) | 100.7(16.5) | 99.87 (16) | 0.78 | |
| Placebo | 97.6 (14) | 96.4 (12.5) | 95.5(13.4) | 95 (13) | |||
| AST | Spinal-Z | 31.5 (14.8) | 31.2 (13.8) | 33.5 (13.5) | 0.3 | ||
| Placebo | 28.1 (14.2) | 27.4 (13.2) | 26.7 (13) | ||||
| ALT | Spinal-Z | 26.1 (13.4) | 25.7 (13) | 26.5 (13) | 0.57 | ||
| Placebo | 24 (10) | 23.9 (10) | 23.7 (9.2) | ||||
| Alkaline phosphatase | Spinal-Z | 228.4 (22.5) | 226.6 (20.2) | 226.9 (18) | 0.44 | ||
| Placebo | 231.7 (14.2) | 231.1 (12.8) | 234.6 (35.7) | ||||
| Total bilirubin | Spinal-Z | 1 (0.17) | 1 (0.16) | 1.01 (0.13) | 0.87 | ||
| Placebo | 1.01 (0.17) | 0.99 (0.15) | 1.01 (0.13) | ||||
| Direct bilirubin | Spinal-Z | 0.45 (0.09) | 0.45 (0.09) | 0.45 (0.07) | 0.3 | ||
| Placebo | 0.44 (0.11) | 0.43 (0.1) | 0.45 (0.09) |
aThe values are expressed as mean (SD).
bP value for group-time interaction based on the results of GEE analysis.
| Gastrointestinal Problems | Week 0 | Week 2 | Week 4 | Week 16 | Week 24 | P Valueb | |
|---|---|---|---|---|---|---|---|
| Nausea and vomiting | Spinal-Z | 0 | 7 (18.4) | 6 (15.8) | 0 | 0 | 0.22 |
| Placebo | 0 | 0 | 0 | 0 | 0 | ||
| Dysphagia | Spinal-Z | 24 (63.1) | 24 (63.1) | 26 (68.4) | 14 (36.8) | 7 (18.4) | < 0.05 |
| Placebo | 21 (55.2) | 21 (55.2) | 21 (55.2) | 23 (60.5) | 24 (63.1) | ||
| Poor appetite | Spinal-Z | 28 (73.6) | 33 (86.8) | 35 (92.1) | 14 (36.8) | 10 (26.3) | < 0.05 |
| Placebo | 26 (68.4) | 22 (57.8) | 15 (39.4) | 25 (65.7) | 25 (65.7) | ||
| Stomachache | Spinal-Z | 35 (92.1) | 33 (86.8) | 26 (68.4) | 8 (21.05) | 8 (21.05) | < 0.05 |
| Placebo | 35 (92.1) | 35 (92.1) | 35 (92.1) | 34 (89.4) | 34 (89.4) | ||
| Itching | Spinal-Z | 0 | 3 (7.9) | 2 (5.83) | 0 | 0 | 0.31 |
| Placebo |
aThe values are expressed as No. (%).
bP value for group-time interaction based on the results of GEE analysis.
5. Discussion
This randomized controlled clinical trial was performed to evaluate the effects of Spinal-Z, a capsule containing a mixture of herbal extracts, on blood parameters and gastrointestinal symptoms and to study its potential side effects in patients with metastatic gastric adenocarcinoma. Peganum harmala as one of the 2 constituents of Spinal-Z is known to reduce proliferation and affect cell lysis in cancerous cell lines (9).
The leaf extract of D. kotschyi can inhibit tumor proliferation in mice. Spinal-Z and its constituents can reduce the viability of cells in a dose-dependent manner (4). From a pharmacological viewpoint, Peganum harmala has antibacterial effects against microorganisms such as Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumoniae (10), as well as ameliorative effects on the cardiovascular system (eg, antispasmodic, anticholinergic, antihistaminic, and antiadrenergic effects) (11). However, in the present study, this agent had no significant effects on the cardiovascular or serological system.
Seeds and roots of Spinal-Z are reported to have antifungal and antileishmanial activities (12, 13). In this study, this agent could significantly decrease FBS level (not in comparison to the control group). However, we could not find any similar studies for comparison. Spinal-Z could significantly increase the patients’ appetite and reduce dysphagia and stomachache in the follow-up. This finding might be related to the antibacterial or other similar features of this agent on bacteria, fungi, and gut flora. Nevertheless, we could not find any studies regarding the effect of Spinal-Z or its components on gastrointestinal symptoms.
5.1. Limitations
Some limitations of the present study should be acknowledged. Spinal-Z is only available in Iran and we could not find any other studies investigating the effects or side effects of this agent. In addition, lifestyle, eating habits, and environmental factors can strongly affect gastrointestinal complications. Therefore, caution should be taken while interpreting the results. Further research with a larger sample size about the effect of Spinal-Z is recommended.
5.2. Conclusion
Spinal-Z does not have any adverse effects on cardiovascular, renal, and hematopoietic systems or blood glucose level. Spinal-Z as an accessible and affordable medication with very few side effects may be a suitable option for treating complications in patients with metastatic gastroesophageal adenocarcinoma.