1. Background
Bone remodeling is achieved via bone resorption by osteoclast cells on one side and the formation of new bone by osteoblast cells on the other side; which is continued during life (1, 2). The increase in bone resorption markers such as C-terminal telopeptide of type 1 collagen (CTX), pyridinoline, and hydroxyproline and the decrease in biochemical markers of bone formation such as alkaline phosphatase and osteocalcin are associated with osteoporosis or bone destruction (2). This process is associated with aging by gradually decreasing bone mass and osteoporosis (3). However, several factors of clinical disorders such as hyperthyroidism digestive disorders, genetics, lack of mobility, physical inactivity, and increased serum cortisol affect this phenomenon (4).
On the other hand, cigarette smoking reduces the absorption of calcium, which is a determinant of bone formation (5). Smoking reduces the density of minerals, the absorption of calcium along with hyperparathyroidism while increases bone resorption. Also, the findings of a study on twin females showed that those who consumed more cigarettes had lower levels of bone density compared with those who had less cigarette smoking (6). In this regard, the findings of some studies indicated that osteocalcin concentration, as a marker of bone formation, was decreased in female smokers (7). Some findings also suggest that levels of CTX and dihydroxyproline, which are the main characteristics of bone resorption or formation, are higher in smokers than non-smokers (8).
These studies suggest that, even after matching for age and body weight, smokers have a lower bone density than non-smokers (9). Hence, the creation of appropriate strategies to prevent or reduce osteoporosis in smokers is at the heart of the attention of health researchers. Physical activity, in this regard, is one of the important determinants of bone mass and its role in the skeletal tissue and the bone mass increase has been numerously reported. As bone mass is decreased in smokers, the increase in strength and physical activity is associated with more bone mass (10). Although the role of exercise and physical activity on bone metabolism as well as bone marker indices in smokers have been less studied, several studies have been conducted on healthy and sick non-smoker populations, which are often contradictory and inconsistent. For example, in the study conducted by Evans et al. (11), 9 months of moderate aerobic training with moderate intensity led to a reduction in serum CTX levels without alteration in alkaline phosphatase in postmenopausal females. In contrast, in another study, Tosun et al. (12), there was no change in osteocalcin and other indicators of bone formation and bone resorption following exercise in healthy females. Also, some studies Courteix et al. (13) and Jaffre et al. (14) have pointed out higher levels and normal levels (15) of bone destruction indicators such as CTX in female athletes. Another study demonstrated that in sports such as volleyball and basketball that are weight-bearing, bone formation, and bone density are much higher than sports that are not weight-bearing (16). A review of the available pieces of evidence suggests a contradiction in the response or compatibility of bone-marker indices in healthy and patient populations. On the other hand, the type of population studied and other factors, including exercise intensity, type of exercise protocol such as strength or endurance (17), and the number of exercise sessions (18) are of special importance on how exercise activity affects bone remodeling in both healthy and patient populations.
Given the contradictory evidence about the role of exercise and physical activity depending on intensity, frequency or duration of training protocol on bone metabolism, as well increasingly cigarette smoking among males in our country and its relation with osteoporosis, our study was conducted with the mentioned aims. On the other hand, most studies in this area have been done on females and there are limited studies on inactive males with cigarette smokers particularly in our country.
2. Objectives
The present study aimed to assess the effect of 12 weeks of aerobic training program on serum CTX levels as one of the most important indicators of bone resorption as well as on serum calcium levels in sedentary adult male smokers.
3. Methods
3.1. Human Subjects
The participants were comprised of thirty-two sedentary adult male smokers that matched for sex (males), age (42 ± 7, aged), and weight (94 ± 10 kg) that participated in study by convenience and purposive sampling and selected to either exercise group (3 months aerobic training, 3 times/weekly at 60% - 75% of maximum heart rate, n = 16) or control group (no exercise, n = 16) based on random sampling using a table of random numbers (Winter 2016, Saveh, Iran). The study protocol was approved by the Ethics Committee of Islamic Azad University, Saveh branch, Iran. After the nature of the study was explained in details, informed consent was obtained from all subjects.
3.2. Inclusion Criteria
The main inclusion criteria for participation in this study were cigarette smoking at least for 3 years, non-athletes and non-alcoholic. The participants had not previously regular exercise or any previous participation in weight loss programs (at least during the last 6 months). None of the participants used medication therapies for osteoporosis or other disorders, and none of them had an injury history that would prevent exercise (19).
3.3. Exclusion Criteria
Those with a history of chronic diseases (asthma, metabolic syndrome, diabetes, cardiovascular or other diseases) were also excluded from the study (19).
3.4. Anthropometry
Height and body weight were measured to the nearest 0.1 kg and the nearest 0.1 cm, respectively on the same day. The BMI was calculated as the weight (kilograms) divided by the square of the height (meters). Hip and abdominal circumferences were measured in the most condensed part. Body fat (%) and visceral fat were measured using a body composition monitor (OMRON, Finland). Each measurement was conducted 3 times and the average was recorded.
3.5. Exercise Program
Aerobic training lasted 3 months as three sessions weekly, consisting of a warm-up then a 45 - 60-minutes treadmill exercise at 60% - 80% HRmax followed by a 5 - 10 minutes cooling-down. In each session, the main exercise was running with no slope ((20), Modified). The heart rate was determined by polar telemetry to calculate the exercise intensity. At the first week, the exercise intensity was 60% HRmax that gradually was increased to 80% HRmax at last week of the training program. Control subjects were instructed to maintain their habitual activities throughout the study. All participants were instructed to maintain their usual diet throughout the study.
3.6. Blood Analyses
Blood samples were obtained before of training program and 48 h after last exercise session with regard to measuring calcium and CTX of all subjects. Samples were taken following a 10 - 12 overnight fast between 8:00 A.M. - 9:00 A.M. All participants were banned any heavy physical activity for 48 hours before sampling. To separate serum, samples were dispensed into EDTA-coated tubes and centrifuged, then aliquots were stored at -80ºC until biochemical analyses. The ELISA method (enzyme-linked immunosorbent assay for quantitative detection of human CTX, Biovendor, Austria) was used to measuring serum CTX. The inter and intra-assay coefficients of variance and sensitivity of the test were 3.0%, 10.9%, and 40 mg/mL, respectively. Serum calcium was analyzed by a chemical colorimetric method using spectrophotometer (Biochemistry Company, Iran).
3.7. Statistical Methods
All statistical analyses were performed using SPSS (version 16 .0; SPSS Inc, Chicago, IL, USA). Given the normal distribution of the data, which was analyzed by Kolmogorov-Smirnov normality test, subsequent analysis was independent and paired sample t-test. At baseline, independent t-test was used to compare all variables between the two groups. Pre- and post-training differences of all variable were assessed by paired t-test in each group. The changes less than 0.05 were considered statistically significant.
4. Results
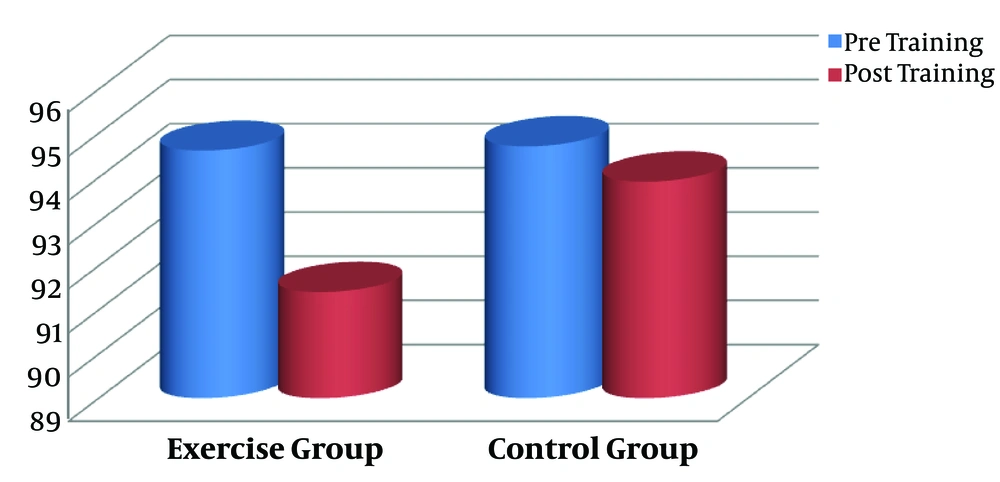
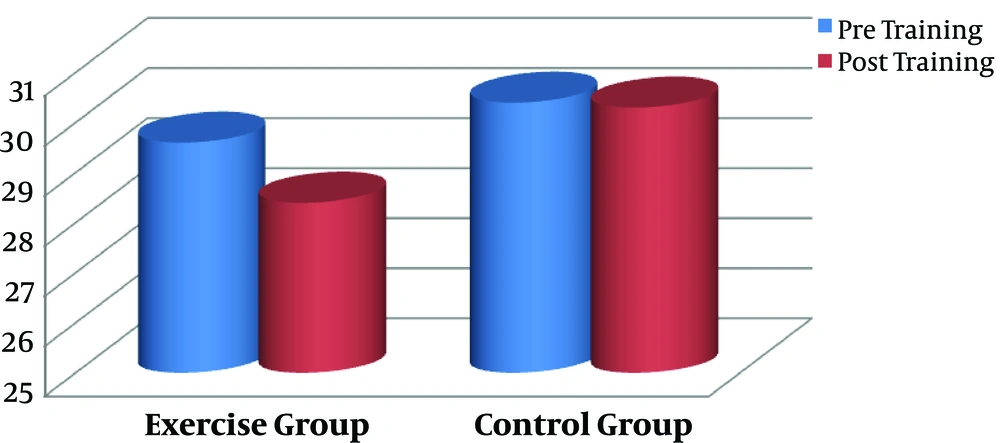
In the present study, we aimed to assess the impact of 3 months aerobic training on CTX as a resorption bone marker and calcium in male smokers. Pre- (baseline) and post-training anthropometrical markers of the groups are shown in Table 1. There were no statistically significant differences between the exercise and control subjects with regard to the anthropometrical indices at baseline (P > 0.05). The aerobic intervention resulted in significant decreases in weight (P = 0.000, Figure 1), body fat percentage (P = 0.000, Figure 2), and the other anthropometrical indices in the exercise group compared with the control subjects (Table 1).
| Variables | Exercise Group | Control Group | ||||
|---|---|---|---|---|---|---|
| Pre-Training | Post-Training | P Value | Pre-Training | Post-Training | P Value | |
| Age, y | 41.6 ± 7.39 | 41.6 ± 7.39 | - | 42.1 ± 2.81 | 42.1 ± 2.81 | - |
| Height, cm | 175 ± 4.4 | 175 ± 4.4 | - | 175 ± 3.6 | 175 ± 3.6 | - |
| Weight, kg | 94.6 ± 6.25 | 91.4 ± 6.50 | 0.000 | 94.7 ± 9.79 | 93.9 ± 9.81 | 0.433 |
| AC, cm | 106 ± 6.3 | 101.8 ± 5.67 | 0.000 | 105 ± 7.9 | 104.6 ± 8.25 | 0.362 |
| HC, cm | 105 ± 2.49 | 100.7 ± 2.49 | 0.000 | 104 ± 6.13 | 104.1 ± 6.30 | 0.562 |
| Body fat, % | 29.6 ± 2.44 | 28.4 ± 2.16 | 0.000 | 30.4 ± 2.73 | 30.3 ± 2.89 | 0.463 |
| BMI, kg/m2 | 30.9 ± 2.41 | 29.8 ± 2.42 | 0.000 | 30.7 ± 2.59 | 30.6 ± 2.67 | 0.384 |
Abbreviations: AC, abdominal circumference; AH, hip circumference; BMI, body mass index.
Table 2 represents the pre- and post-training circulating levels of CTX and calcium of the two groups. Based on independent t-test, significant differences were not observed in these variables between the groups at the baseline level (P > 0.05).
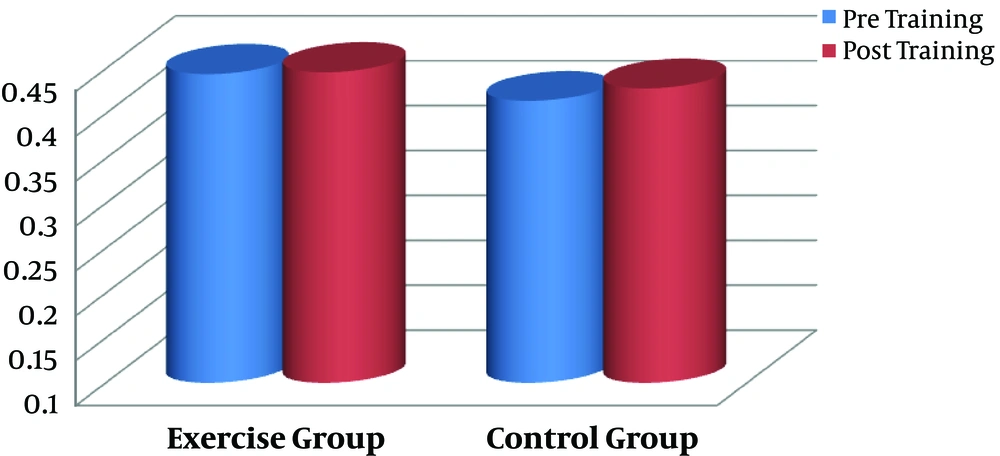
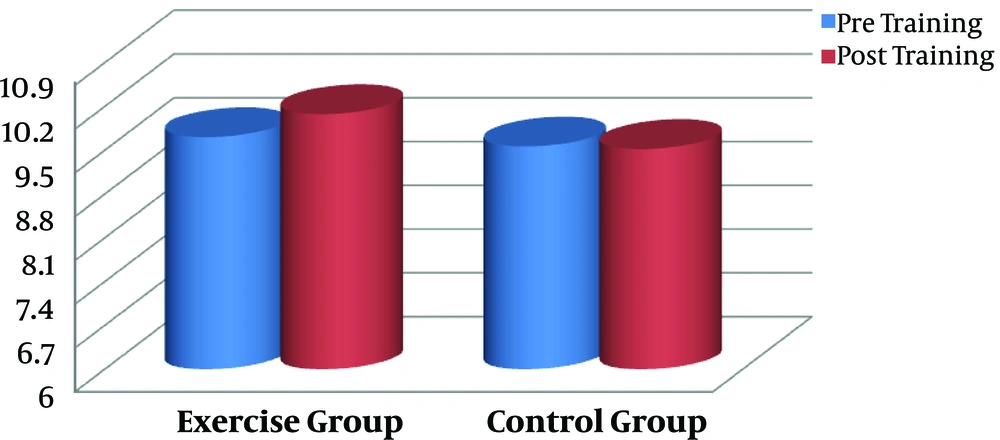
The effect of aerobic training on serum CTX was the main aim of the present study. Data by paired sample t-test showed no significant difference between pre- and post-training values in serum CTX of the exercise subjects (P = 0.835, Figure 3). No significant difference was also found in calcium concentration by aerobic intervention in comparison to baseline level in the exercise subjects (P = 0.126, Figure 4). There were no changes in variables in the control group (P > 0.05).
| Variables | Exercise Group | Control Group | ||||
|---|---|---|---|---|---|---|
| Pre-Training | Post-Training | P Value | Pre-Training | Post-Training | P Value | |
| CTX, U/L | 0.444 ± 0.202 | 0.446 ± 0.209 | 0.835 | 0.414 ± 0.091 | 0.428 ± 0.076 | 0.905 |
| Calcium, mg/dL | 9.71 ± 0.47 | 10.08 ± 0.51 | 0.126 | 9.56 ± 0.14 | 9.52 ± 0.14 | 0.486 |
Abbreviation: CTX, C-terminal telopeptide of type 1 collagen.
5. Discussion
No significant change in serum CTX between the two groups is the main finding of this study. In other words, 12 weeks of aerobic training, 3 sessions per week did not lead to significant changes in CTX levels in adult male smokers. In addition, calcium levels did not change significantly in response to training intervention. The lack of difference in these variables in smokers is reported while the research background on the response or compatibility of these bone markers in smokers is limited. However, the findings of other studies in response to exercise training in non-smoker populations are somewhat contradictory. For example, in a recent study by Toriola et al. (21), a weight loss program of 6 and 12 months in the form of diet modification and exercise training was associated with lack of CTX change in obese or overweight females with breast cancer. In another study Szulc et al. (8), 6 months aerobic exercise, 3 sessions per week, did not significantly change in alkaline phosphatase and calcium levels in middle-aged females. However, in the study by Kilebrant et al. (22), 6 months of total body vibration (42 - 40 Hz, 15 - 5 min), two sessions per week, to strengthen bone mass markers and bone turnover of 5 to 16 years old children with mental disability, resulted in a significant decrease in CTX compared with the baseline levels. Likewise, in a study by Evans et al. (11), 9 months of moderate-intensity aerobic training led to a reduction in serum CTX levels in postmenopausal females. Ghasemalipour et al. (19) reported a significant decrease in serum CTX by 3 month-aerobic training in adult males with mild to moderate asthma.
CTX has been introduced as an osteoclast-derived bone destruction marker, and its increase alongside the fixed levels of osteocalcin is a marker of bone destruction (23). On the other hand, scientific sources refer to impaired bone formation markers or bone destruction in smokers (6). In other words, the increase in nicotine induced by smoking is associated with impaired bone metabolism (8). In this regard, it has been shown that nicotine affects the formation of bone due to the damage to the signaling pathways and metabolism associated with the extracellular matrix (24). It has also been reported that Gao et al. (25) the exposure of male Wistar rats to cigarette smoke for 4 months, due to an increase in the levels of osteoclasts and decreased volume of bone connective tissue, the number and thickness of the connective tissue bands, the rate of bone formation, and the reconstruction of osteoblasts reduced the process of bone turnover. The increase in reactive oxygen species and free radicals due to smoking is also associated with the inhibition of the formation of osteoblasts in the bone surface (26). The adverse effects of cigarette smoking and its cessation on the prevalence of osteoporosis have also been reported by some other studies (27-29). Smoke-induced hypercortisolism, which has been reported frequently, directly affects osteoblasts, osteoclasts, and bone metabolism. The increase in cortisol in response to cigarette smoking is also associated with impairment of calcium reabsorption from the digestive system and kidney tubules (30).
The process of bone formation or destruction does not depend solely on changes in CTX or osteocalcin as indicators of bone destruction or formation. It is an outcome of osteocalcin and CTX changes that determine bone formation or degradation. The increase in the ratio of osteocalcin to CTX has also been shown to improve the profile of bone formation (31). In fact, an increase in the ratio of formation markers to bone destruction markers has been shown to be an indicator of the beneficial effects of therapeutic stimulants on bone turnover. In the study by Karabulut et al. (32), 6 weeks of low intensity and intense resistance training led to an increase in the ratio of alkaline phosphatase to CTX in elderly people. It should be noted that similar to osteocalcin, alkaline phosphatase is also an indicator of bone formation. Also, the beneficial effects of exercise depend on the type of exercise program and the studied population. In a recent study, muscular contractions in the form of vibration in children aged from 5 to 16 years with a mental disability led to a significant decrease in CTX and a significant increase in osteocalcin compared with baseline levels (22). Researchers have also noted the beneficial effects of exercise in combination with some effective stimuli on bone formation processes. For example, in the study by Jiang et al. (33), the combination of 12 months of aerobic exercise with statin resulted in CTX reduction in participants with metabolic syndrome. Also, in a recent study by Achiou et al. (34), daily aerobic exercise plus the use of sclerostine antibody for 9 weeks increased the markers of bone formation, such as osteocalcin, and prevented destructive effects of glucocorticoids on bone mineral density, including bone mineral capacity, bone density, and the volume of bone mass connective tissue in male Wistar rats injected with glucocorticosteroids. Sun et al. (35), also revealed that regular aerobic exercises combined with Naringin for 60 days led to an increase in the osteocalcin expression and a reduction in CTX-1 type I in male Wistar rats. Researchers believe that the effects of exercise training on the bone are particularly dependent on the intensity and duration of the exercise; the intensity or load on the bone is more important than the exercise time (36). Also, low-intensity exercise programs cannot prevent osteoporosis. Furthermore, high-intensity exercises are possibly associated with a reduction in the thickness of the trabecular and cortical bones (34). The unchanged levels of CTX and calcium in the present study may be attributed to the moderate intensity of aerobic training; however, the small number of samples, which is a limitation of the current study, might also be in some way the reason for the insignificant findings. In addition, no response measurement of other bone metabolism markers such as osteocalcin, alkaline phosphatase, calcitonin, and parathyroid hormone are the other limitations of this study. Determining the therapeutic effects of exercise training on bone metabolism and prevention of osteoporosis in smokers are remarkable outcomes from a clinical perspective.
5.1. Conclusions
Despite the beneficial effects of exercise training on bone metabolism reported by some previous studies, the level of calcium and CTX remained without change by 3 months aerobic training in our study. It is likely aerobic training affected other bone metabolism determinatives such as PTH, osteocalcin, and alkaline phosphatase as bone formation markers. Therefore, further studies are necessary to elucidate the therapeutic and clinical significance of aerobic training in bone metabolism in smokers and other populations.