1. Background
Fasting in the month of Ramadan is obligatory upon every adult Muslim in good health condition (1). In every Ramadan, about 400 million Muslims out of one million Muslims do this obligation (1). In this month, a person, who wants to fast shall abstain from doing specific actions, such as eating, drinking, and smoking from the morning (before sunrise) to Maghrib (after sunset) (2). This month, having special characteristics, causes changes in food habits, amount of taken calories, sleeping and daily physical activities, which can lead to changes in hunger-satiety hormones (3, 4). Considering the shortage of performed studies related to hunger-satiety hormones in overweight and thin people, a more precise and accurate study is needed to study the effect of fasting on this type of hormones. Some hunger-satiety hormones are ghrelin and peptide YY (PYY) (5-9).
Ghrelin is a peptide produced in the gastrointestinal tract, which is involved in stimulating appetite and decreasing body fat burning (6, 10). The level of ghreline is decreased before and after eating (11). Ghrelin is also produced in hypothalamus cores, which stimulates growth hormone release in the pituitary gland (12). The PYY hormone or peptide YY is one of the appetite regulators, which was recognized as the hormone affecting the digestive system for the first time in 1985 (13). Furthermore, PYY is released from L cells of the digestive system, especially in the ileum and colon, in response to feeding (14). The concentration of this hormone reaches maximum levels, 1 to 2 hours after eating. The maximum level depends on the amount of taken calories and fat. The PYY hormone decreases appetite by affecting the brain. It decreased the movement of the digestive tract, which in turn increase electrolytes intake in the large intestine. The PYY hormone fights against ghreline effects (7). It is reported that fasting in Ramadan causes a decrease in PYY concentration up to 30%. However, no significant relationship was reported between PYY changes and insulin and leptin changes (15). Decrease in PYY concentration in the morning (fasting) can be because of changes in day and night cycles.
According to evidence, fasting in Ramadan causes changes in hunger-satiety hormones (3, 4). Ganjali et al. performed a study in 2016 about the effect of fasting on the levels of glucose, lipid profiles, adiponectin, and leptin on 24 overweight males and normal weight females and found that body weight, BMI, and cholesterol were decreased and HDL-C and ratio of cholesterol to high density lipoprotein in the fat group were increased remarkably, while the level of adiponectin and leptin was not changed in considerable amounts (3). In another research by Attarzadeh Hosseini et al. on the effect of fasting accompanied by physical exercises (with intensity of 50% to 65% of maximal heart rate, 3 sessions per week for 60 minutes) on 27 obese females aged 20 to 45 years, it was found that the level of leptin at the end of Ramadan was decreased remarkably and adiponectin was increased significantly (4). Faraji et al., through studying the effect of sport activities on the plasma level of PYY and NPY in obese males, found that one session of sport activities increases the plasma level of PYY and NPY remarkably in comparison with their level before sport activities (16). In summary, the contradictory results of the performed studies about the effects of fasting on hunger-satiety hormones, as well as the lack of studies about the effects of fasting in Ramadan on hunger-satiety hormones, especially ghrelin and PYY, and the occurrence of Ramadan in summer and long hours of fasting, led the current authors to study fasting in Ramadan. Therefore, considering the importance of fasting on hunger-satiety hormones in obese and thin females and comparing these 2 groups can help reach useful achievements in relation to appetite in Ramadan. It also helps achieve a better understanding of the physiological conditions of this group of the society in Ramadan.
2. Objectives
Fasting leads to a weight loss as it involves 2 relatively light meals (known as “Iftar” and “Suhur”). In other words, while fasting, the amount of calories taken is less than required and to make up, one’s body begins to use the stored fat, which causes a weight loss. However, if the amount of food taken in suhur, iftar or from iftar to suhur time is as much as the body requires or even more, people gain excessive weight due to limited physical activity. As it is important to understand how fasting affects the hunger-fullness cycle, changes in body weight, BMI, and percentage of fat from a weight regulatory perspective, the present research explored the effect of fasting on appetite regulatory hormones in obese and thin females.
3. Methods
The present research was performed using the semi-experimental method by repetitive measurement of 2 experimental groups (obese and thin females) during the Ramadan of 2017 (July and August). The statistical population of this research were obese females (with body mass index of more than 30 kg/m2) and thin females (with body mass index of between 18 and 20 kg/m2), selected using the purposive availability sampling method. In the first stage, the population became familiar with the nature and manner of the cooperation for the research performance.
Inclusion criteria for participating in research were being in sound health, according to the health questionnaire, having no medicine, drug or tobacco intake, and not taking part in any exercise programs (at least 2 months before the research). The exclusion criteria in the research included having uncontrolled hypertension or malignant hypertension, having severe neurological disease (depression, schizophrenia, and addiction), having severe medical disease (diabetes, hyperlipidemia, cancer, and rheumatologic disorder), being pregnant or lactating, having swallowing problems, under therapeutic regime, having history of at least 15 days of fasting during the 2 months before Ramadan, having at least 5 days not fasting during Ramadan. The population upon testing participated in the research voluntary and signed the letter of consent. The population was then allocated to 2 groups of obese (12 females) and thin (13 females) individuals. The following equation was used to determine the sample size:

In this equation, the power of the test was 0.8, a = 0.05, and variation of means was 5 units. Based on the estimated equation, a sample size of 10.97 was obtained. For more caution, 25 females were included in the 2 experimental groups.
In addition, the steps for tests were approved by the ethics committee of Medical research at the faculty of Payam-e Noor University of Mashhad under the code 1221.18282 and carried out in February 2017 at Payam-e Noor University of Mashhad.
3.1. Body Composition
The body mass index (BMI) was measured by height and weight values as follows: weight (kg)/height (m2). The body composition was determined by bioelectric impedance using in body-720 (made in South Korea) to study fat mass (FM). The height of the same person was measured using an electronic balance with a stadiometer (SECA-Germany) to the nearest 0.1 cm.
3.2. Blood Samples
In this research, for examining the parameters of this study, 5 mL of venous blood was obtained at 4 occasions from each subject; the first session took place 3 days before Ramadan, the second session after 14 days of fasting, the third session after 28 days of fasting, and the fourth session 2 weeks after Ramadan. The time of blood sampling in the study was 3:00 to 4:00 PM, at which all volunteers were fasting. The concentration of serum ghrelin and PYY was measured via the enzyme linked immunosorbent assay (ELISA) kit test (Mark Eastbiopharm, made in china, under licence of America) with sensitivity of intra-assay: CV < 10%.
3.3. Data Analysis
All values are presented as mean ± standard deviation (SD). The collected data was analyzed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). For normally distributed data, the Shapiro-Wilk test was used and comparing variances was done via Levene’s test. The differences between the groups were evaluated using the one-way analysis of variance (ANOVA) test. Significant differences were considered at P ≤ 0.05.
4. Results
The findings of the present study are summarized in Tables 1 and 2. The characteristics of the participants in the study of the 2 groups of obese and thin individuals are shown in Table 1. There was no significant difference between the 2 groups in terms of age, height, weight, and body mass index before the intervention (P < 0.05).
| Variables | Obese (N = 12) | Thin (N = 13) |
|---|---|---|
| Age, y | 44.50 ± 4.90 | 35.84 ± 8.99 |
| Height, cm | 157.00 ± 6.88 | 162.15 ± 5.27 |
| Weight, kg | 82.88 ± 8.60 | 53.53 ± 5.91 |
| BMI, kg/m2 | 33.62 ± 3.43 | 20.31 ± 1.32 |
aData presented as mean ± SD.
| Variable | Stages | Variations | ||||||
|---|---|---|---|---|---|---|---|---|
| Week Before Ramadana | Day 14 of Ramadana | Day 28 of Ramadana | Week After Ramadana | P Valueb | P Valuec | |||
| F | P Value | F | P Value | |||||
| Weight, kg | 96.18 | 0.01c | ||||||
| Obese | 82.88 ± 8.60 | 81.70 ± 8.44 | 81.15 ± 8.37 | 82.03 ± 9.34 | 5.68 | 0.03c | ||
| Thin | 53.53 ± 5.91 | 52.90 ± 5.80 | 52.70 ± 5.90 | 53.59 ± 5.83 | 11.92 | 0.01c | ||
| BMI, kg/m2 | 156.82 | 0.01c | ||||||
| Obese | 33.62 ± 3.43 | 33.19 ± 3.49 | 32.96 ± 3.46 | 33.31 ± 3.75 | 4.29 | 0.02c | ||
| Thin | 20.31 ± 1.32 | 20.06 ± 1.32 | 19.99 ± 1.37 | 20.30 ± 1.33 | 10.06 | 0.01c | ||
| Body fat percent, % | 48.36 | 0.01c | ||||||
| Obese | 45.43 ± 5.27 | 44.39 ± 5.46 | 43.71 ± 5.32 | 44.9 ± 5.01 | 9.35 | 0.02c | ||
| Thin | 30.40 ± 5.61 | 29.33 ± 5.65 | 28.56 ± 5.96 | 30.29 ± 5.41 | 4.25 | 0.04c | ||
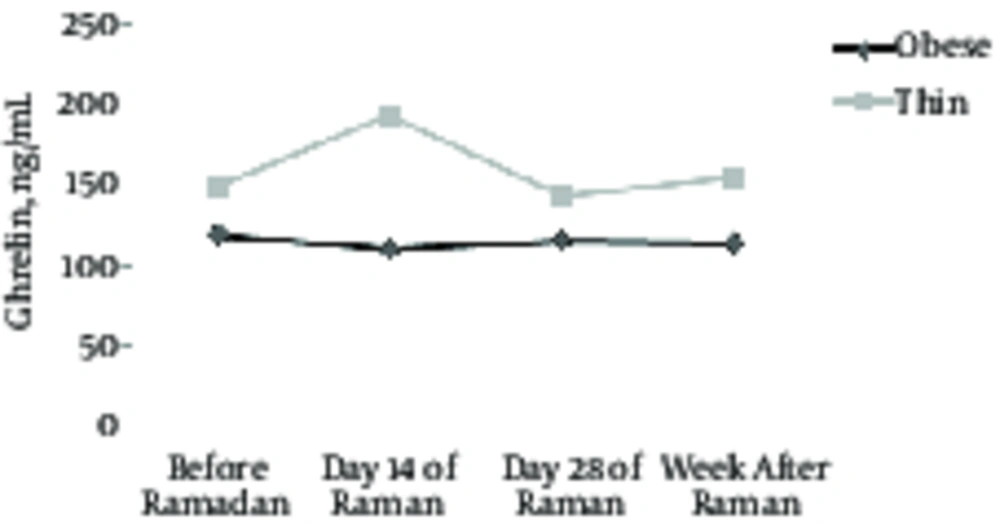
| Ghrelin, ng/mL | 0.59 | 0.45 | ||||||
| Obese | 119.00 ± 45.05 | 109.80 ± 31.8 | 115.60 ± 57.40 | 114.00 ± 44.51 | 3.02 | 0.095 | ||
| Thin | 147.75 ± 20.71 | 192.80 ± 21.20 | 142.19 ± 22.53 | 153.65 ± 21.94 | 0.19 | 0.73 | ||
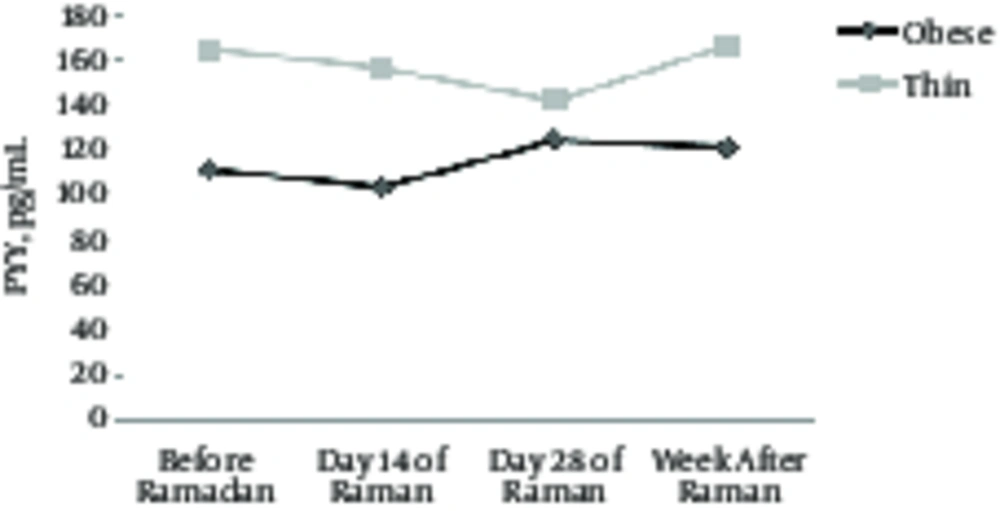
| PYY, pg/mL | 0.43 | 0.51 | ||||||
| Obese | 111.20 ± 52.5 | 103.50 ± 42.15 | 124.60 ± 73.82 | 120.95 ± 59.7 | 0.65 | 0.59 | ||
| Thin | 164.80 ± 32.91 | 156.35 ± 33.20 | 142.10 ± 33.09 | 166.80 ± 33.16 | 0.47 | 0.70 | ||
aData presented as mean ± SD.
bP value within group.
cP value between group
dA significant level P < 0.05.
According to Table 2, the current results showed a significant weight loss, BMI, body fat percentage in the two groups by the end of Ramadan (P < 0.05). However, Differences within groups in ghrelin and PYY levels did not change significantly (P > 0.05) (Figures 1 and 2). Differences between groups were significant in weight, BMI, and body fat percentage (P < 0.05). Variation in variance between groups, such as ghrelin and PYY levels, was not significant (P > 0.05).
5. Discussion
The aim of this study was to investigate the effect of fasting on appetite-regulating hormones in obese and thin females. During Ramadan, type and amount of calories intake and sleep habits may change. These changes can affect size, body composition, and hunger-satiety hormones. According to the results of the present study, 1 month of fasting does not cause any significant change in the level of serum ghrelin.
The results of this study are consistent with the findings of Saremi et al. and Martinez et al. (17, 18), yet are inconsistent with the findings of Cole et al., Khalilzadeh et al., and Molanovruzi et al. (19-21). Saremi et al. reported that 12-week aerobic exercise on obesity led to a significant decrease in weight, BMI, and body and abdominal fat. Also, serum level of ghrelin and leptin did not change in response to aerobic exercises (17). Martinez et al. noted that insulin level was decreased, yet levels of ghrelin, leptin, and PYY3 showed no significant change (18). On the other hand, Cole et al. investigated the effect of high intensity exercise on energy balance and ghrelin plasma of 18 males (9 obese males and 9 normal males), with average age of 29 years, and found that ghrelin levels in obese males was greater than normal individuals. Exercise was more effective in the normal group than the obese group (21). Khalilzadeh et al. investigated the effect of 8 weeks of aerobic exercise with low or moderate intensity on appetite, activity energy cost and changes of plasma acylated ghrelin of 16 obese and thin volunteers and found that the feeling of hunger and the amount of plasma acylated ghrelin were increased in the 2 groups during the experiment (19). Molanovruzi et al. showed no significant changes on feeling of hunger, the amount of plasma acylated ghrelin, leptin and insulin plasma (20). These results can be because of metabolic changes arising from different food habits, type of foods, intensity of exercise, weather, as well as decrease in the volunteers’ metabolism at rest (22) and major fluctuation in hormones (23). It seems that food intake is under control of nervous and endocrine systems. Ghrelin is a hormone that regulates food intake and functions as a neuropeptide in the central nervous system. The exact mechanism is not clear (24). On the other hand, evidence shows that in human, sleep has an important role on regulation of the ghrelin hormone, in a way that sleep shortage causes a dramatic increase in ghrelin levels. These changes are accompanied by increase of appetite and hunger (25). These changes were approved by large population studies. For example, in a performed study on 1024 samples, it was found that the leptin level was low and ghrelin level was high in people, who had a bad quality sleep (5 hours in a day) in comparison with people, who enjoyed good quality sleep (8 hours a day). These changes are accompanied with an increase of desire for eating and researches know it is a justification for high BMI in people, who experience sleep disorders (26). These results probably mean that people with low quality sleep have high level of ghrelin and low level of leptin. This hypothesis shows that sleep disorders are accompanied by appetite increase and hunger (27). In other words, these hormone changes may affect weight increase and sleep disorders. In this research it was hypothesized that fasting can be effective in obesity treatment, not only for less calorie intake, but also for its result on Adipocyte fall. Nonetheless, this hypothesis can be rejected for several reasons; 1, the body needs more sleep for regulating and expressing ghrelin hormone and Ramadan is not an enough time. 2, Considering the fact that other nervous and hormonal factors, such as orexin affect appetite and food intake, sleep changes arising from fating in Ramadan, may change appetite from other routs, challenging more studies.
In this research it was shown that one month of fasting had no considerable effect on concentration of serum Peptide YY of obese and thin females.
The results of this study are consistent with the findings of Larson-Meyer et al. and Ueda et al. (28, 29). However, it is inconsistent with the findings of Faraji et al. (16). Larson-Meyer et al. reported that the levels of PYY were not increased after running for 60 minutes with 70% intensity and the highest level of oxygen intake (29). Also, based on one study, Ueda et al. did not report any considerable change in plasma levels of PYY of middle-aged females after 60 minutes of exercise on an Ergometer bike with 70% intensity and the highest level of oxygen intake (28). Faraji et al. examined the effect of 7 resistance exercise stations with 60% intensity (one time) followed by 60 minutes of cycling at 65% of maximal heart rate on 24 obese males and found that exercise significantly increased PYY and NPY levels (16). This hormone is involved in increasing energy consumption while decreasing appetite. However, other hunger hormones do not have such an effect (30, 31). In fact, PYY is released from cells in the ileum and by binding to the Y2 receptor, affects the hypothalamus, which suppresses appetite, decreases the energy intake, and increases energy consumption (32). However, the mechanism of PYY increase was not studied in fasting in any research, yet PYY level will increase under effects of signals like gastric acid, cholecystokinin, bile salt, insulin-like growth factor, and calcitonin gene-related peptide and it will decrease under the effect of glucagon-like peptide-1 (33). As it was noted, PYY applies its appetite suppressant effects by g protein receptors, including Y1 to Y6; of them Y2 in central nervous system has more tendency to bind to PYY (31). Although, in the present research the exact mechanism of appetite suppression as a result of increase of PYY hormone during fasting in Ramadan was not studied, previous studies showed that appetite suppression as a result of increase of PYY hormone after exercise is related to the effect of this peptide on Y2 receptors in the hypothalamus and through these receptors, the expression of NPY is decreased thus controlling the synthesis of appetite-stimulating hormone or NPY in the hypothalamus (34). The present research had certain limitations, such as divergent nutritional diets and adaptation responses, limited number of testes (as some refused to participate), and individual differences. Therefore, caution must be taken in interpreting and generalizing the results. Fasting during Ramadan causes physiological changes and can thus influence changes in ghrelin and PYY hormones. As changes in these variables differ among people depending on the length of days, season of year, changes in dietary habits, sleep-wake cycle and physical preparation, coaches and physicians are recommended to consider these issues and think of proper solutions accordingly.
5.1. Conclusion
It could be concluded that, according to the achieved results, no significant changes were seen in ghrelin and serum PYY levels in the 2 groups of obese and thin females at different times of Ramadan. Some other factors, such as different seasons of the year, duration of fasting, food habits, gender, the body fat of the participants, and racial factors may be affective in interpretation of results.