1. Background
HCV is one of the most important causes of liver cirrhosis, end-stage liver disease (ESLD), and hepatocellular carcinoma (HCC) (1). Traditionally, in the last decades, this virus has been treated with interferon-based regimens with or without ribavirin, which was difficult to tolerate due to high complication rates and sub-ideal results (2-4). Since 2010, the direct-acting agents (DAAs) have introduced to the market to gradually replace peg-interferon-based regimens due to the high success rate and safety profile and that it is much more tolerable and acceptable (5-10).
In 2013, sofosbuvir was introduced to the market as a DAA and was able to revolutionize the HCV eradication protocols to shorten the courses with lower complication rates in comparison with protease inhibitor-based regimens. The safety and high success rate of this agent led to the rapid universalization of its acceptance as a unique base for any designed protocol in this regard (11). sofosbuvir is an HCV NS5B nucleotide polymerase inhibitor; when prescribed in combination with ribavirin, it can be effective against different genotypes of HCV (12). This medication has been evaluated in a large number of clinical trials and generally, its safety and tolerability have been proven (13). In addition to a favorable pharmacologic profile and rare cross-reaction with other medications, there has not seen any kinetic effect on the patient diet. sofosbuvir-containing regimens have a high barrier to resistance that suggests sofosbuvir might be an effective anti-viral agent in patients with pre-existing or treatment-emergent NS3/4A or NS5A mutations when combined with other classes of inhibitors. No drug-drug interactions with a variety of drugs, such as cyclosporine, tacrolimus, drugs used in the treatment of HIV infection, methadone, or combined oral contraceptives containing ethinyl estradiol and norgestimate, have been identified to date (11).
sofosbuvir is a potent HCV inhibitor with a broad genotype coverage. The detection of HCV RNA at the end of therapy with SOF-based regimens is not predictive of treatment failure in the majority of patients and therefore, it would not lead to treatment extension (14, 15). Khuzestan province, in the southwest of Iran, is not only the main center for Iran Oil Company but also an important industrial and geopolitical region. Based on different regional patterns of virological resistance and vacancy of such studies in our region, we designed this cross-sectional study to evaluate the efficacy, safety, and tolerability of a 24-week course of sofosbuvir + ribavirin among different genotypes of HCV with or without cirrhosis in Khuzestan province of Iran.
2. Methods
Overall, from September 2015 to March 2017, 93 HCV patients who referred to the hepatology outpatient clinic of Ahvaz Imam Hospital in Khuzestan province were enrolled in the study. The patients were included regardless of HCV genotype or history of previous treatment failure. The exclusion criteria included pregnancy, BMI of less than 18, co-infection with hepatitis B virus or HIV, advanced renal failure, and decompensated liver cirrhosis.
After explanations by the researchers, all of the participants signed informed consent forms to enter the study. The patient's demographic and clinical data including age, sex, smoking, alcohol consumption, history of previous treatment, and the presence/absence of liver cirrhosis were recorded. At the beginning of the protocol, the complete blood tests including CBC and biochemical profile were done and the genotype of HCV viruses was determined by PCR.
For all of the participants, the 24-week regimen of sofosbuvir 400 mg daily plus ribavirin began twice daily, with doses determined based on the body weight (weight < 75 kg: 1000 mg/day; weight ≥ 75 kg: 1200 mg/day). The definitive endpoint in this study was the sustained viral response (SVR) determined as the negative quantitative PCR (Ampliquality HCV type plus kit, AB Analitica srl, via Svizzera, 16, 35127 Padova, Italy) 12 weeks after the end of the treatment period. The study was approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (RDC-9601).
The safety of the treatment was assessed by looking for any side effect in patients, reviewing the consumed medication during the course of the treatment, analyzing laboratory findings, evaluation of vital signs, and physical examination. The patients were followed within 12 weeks after receiving the last dose. Eventually, any of the possible side effects including anemia, neutropenia, headache, insomnia, fatigue, nausea, and pruritus were recorded.
The statistical analysis was performed by using SPSS version 23. Data were analyzed by descriptive statistics including frequency, mean, standard deviation, and frequency percentage. For analyzing the relationship between quantitative and qualitative data, the analysis of variance and Chi-square test were used. The significance level in the tests was considered at P < 0.05.
3. Results
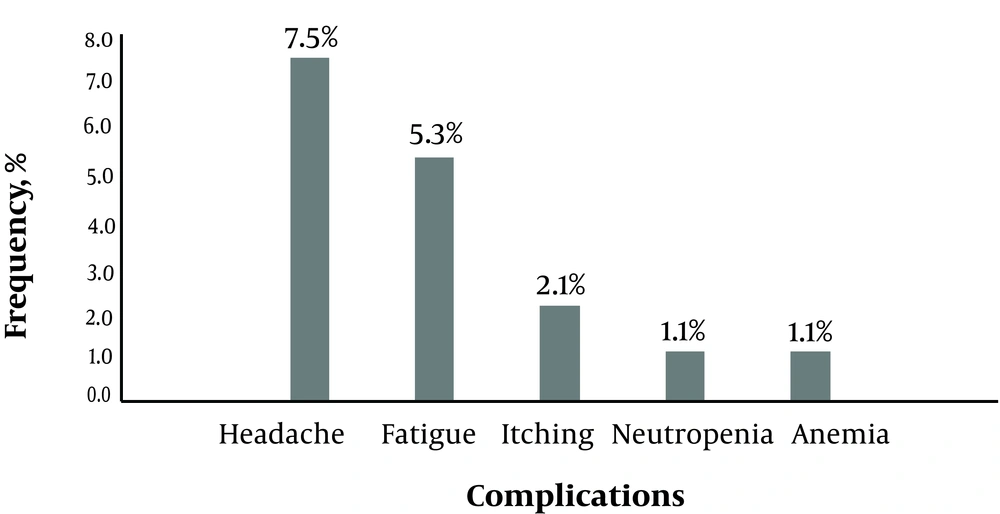
In this study, overall, 93 HCV patients were included. The mean age of the participants was 46.1 ± 12.3 years (range 27 to 80 years). 62 patients (66.7%) were male. The most common genotypes were 1a (51.6%) and 3a (23.7%). The average MELD score of cirrhotic patients was 9.8 ± 2.4 (range 8 to 14). Demographic, clinical, and laboratory results are presented in Table 1. At the end of the study period, 87 patients (93.5%) achieved SVR (ITT 93.5% and PP 96.7%). 16 patients (17.2%) complained of minor side effects without any major complication (Figure 1).
| Characteristics | Result |
|---|---|
| Demographic characteristics | |
| Age, y | 46.1 ± 12.3 |
| Sex (male) | 62 (66.7) |
| Smoking, y | 39 (41.9) |
| Alcoholism, y | 7 (7.5) |
| Concomitant diseases | |
| KTP | 3 (3.2) |
| CKD | 2 (2.2) |
| Treatment history | |
| Naïve | 43 (46.0) |
| Cirrhosis | 26 (28.0) |
| Relapser | 19 (20.4) |
| Non-responder | 5 (5.4) |
| MELD | 9.81 ± 2.45 |
| HCV genotype subtype | |
| non-type | 7 (7.5) |
| *1a | 48 (51.6) |
| *3a | 22 (23.7) |
| *1b | 14 (15.1) |
| *3b | 1 (1.1) |
| *4a | 1 (1.1) |
| Laboratory data | |
| ALT | 51.73 ± 22.60 |
| PLT, 1000/mL | 308 ± 90 |
| Hb, g/dL | 12.47 ± 1.27 |
Abbreviations: CKD, chronic kidney disease; KTP, kidney transplantation; MELD, model for end-stage liver disease; ALT, alanine aminotransferase; PLT, platelet; Hb, hemoglobin.
a Values are expressed as means ± SD or frequencies (%).
Two cirrhotic patients encountered liver decompensation during the course of therapy and stopped their medication. One cirrhotic patient complicated with CVA and was excluded from the study.
There was not any significant relationship between the response to treatment and the age or sex of the participants, the type of HCV genotype, and history of previous treatment (relapser or non-responder) (P < 0.05). The SVR rates for different subgroups of patients based on genotype and the different therapeutic history are summarized in Table 2.
| SVR (N = 87) | No SVR (N = 6) | |
|---|---|---|
| HCV genotype | ||
| 1a | 47 (97.9) | 1 (2.1) |
| *3a | 20 (90.9) | 2 (14.3) |
| *1b | 12 (85.8) | 2 (14.3) |
| non type* | 6 (85.7) | 1 (14.3) |
| *3b | 1 (100) | 0 (0.0) |
| *4a | 1 (100) | 0 (0.0) |
| Treatment history | ||
| Naïve | 41 (95.3) | 2 (4.7) |
| Cirrhosis | 24 (92.3) | 2 (7.7) |
| Relapser | 18 (94.7) | 1 (5.3) |
| Non-responder | 4 (80.0) | 1 (20.0) |
4. Discussion
Although HCV treatment is rapidly evolving, it is necessary to achieve an acceptable regimen with a high rate of efficacy. Despite the availability of sofosbuvir as the first direct-acting agent (DAA) in Khuzestan province for the treatment of HCV infection, limited studies have been conducted on its efficacy on the population of this region and its combination with other agents. Therefore, the present study was conducted with the aim of evaluating the efficacy and safety of sofosbuvir plus ribavirin for the treatment of patients with different genotypes of HCV, with or without liver cirrhosis.
The results of this study showed a high-sustained virological response rate (SVR) (93.5%) in HCV patients with different genotypes, which confirmed the findings of previous studies in North America (5, 13, 16) and Europe (6, 16). In a study from New Zealand for the treatment of HCV with a similar drug combination, SVR was reported about 84%, which is less than SVR in our study (7). Ruane et al. compared the efficacy of sofosbuvir plus ribavirin in Egyptian patients with the chronic HCV genotype 4 infection in two groups who were treated with 12 and 24-week courses. The results showed that 68% of the patients in the 12-week group and 93% of the patients in the 24-week group achieved SVR. The rates of the 12-week course of the mentioned study are lower than our obtained rates while the results of the 24-week course were similar to and consistent with the findings of the current study (17).
Foster et al. in 2015 showed that SVR12 rates in patients with Genotype 3 HCV infection receiving sofosbuvir plus ribavirin for 16 and 24 weeks were 71% and 84%, respectively. These rates were 87% and 100% in patients with HCV genotype 2 (16). In a study by Lawitz et al. among patients with HCV genotypes 2 and 3 and a history of previous treatment, the administration of SOF + Peg-IFN + RBV for 12 weeks showed a high SVR12 rate (89%), regardless of cirrhosis status and without any major complication (13).
On the other hand, the results of a study by Charlton et al. indicated that treatment with sofosbuvir + ribavirin for 24 weeks resulted in SVR12 in 70% of the patients (18). The lack of a consistent response in this study could be explained by performing the evaluation on patients who received a liver transplant and that most of them did not respond appropriately to previous regimens.
The SVR12 rate of 70% was reported in another clinical study, which was based on the dose and duration of treatment with the DAA (19). The reason for the lower efficacy of the DAA could be the co-infection with HIV and HCV in that study. In another study by Osinusi et al. (20), the efficacy of sofosbuvir (400 mg) in combination with two regimens based on patient’s body weight or a fixed low dose of ribavirin (600 mg daily) for 24 weeks in 60 treatment-naive patients with different stages of liver fibrosis was investigated. The results showed that SVR24 was 68% in the body weight-based treated group and 48% in the fixed low dose group. Therefore, the determination of the dose of ribavirin based on body weight was superior and it could be recommended. In that study, 80% of blacks and totally 70% of patients had genotype 1a, and all of the patients had undesirable therapeutic characteristics, which could explain the lower rate of treatment efficacy in comparison with the current study.
The results of Lawitz et al. study showed that SVR12 was 67% for HCV patients treated with either SOF + RBV for 12 weeks or Peg-IFN + RBV for 24 weeks. The rate of SVR12 was seen in 90% of the patients treated with a 12-week regimen with SOF + Peg-IFN + RBV. In the study of Lawitz et al. (21), the response rate (SVR12) in the SOF + RBV group was lower in genotype 3-infected patients than in patients with genotype 2 (56% versus 97%, respectively). It seems that the response rate of treated patients with type 3 genotype can be increased by adding peginterferon to the sofosbuvir-ribavirin regimen or by prolonging the course of the treatment with the sofosbuvir-ribavirin (21). Therefore, low SVR in the study of Lawitz et al. compared to the present study can be attributed to a shorter treatment period and no history of previous treatment in patients of the current study.
In the current study, the percentage of SVR12 was higher than in other studies. The differences between populations in terms of immune status could be an explanation for the higher SVR rate. Moreover, the probability of a lower response to treatment in some studies may be due to the S282T mutation, which was not studied in any of these studies (22).
In the current study, there was no significant correlation between SVR and genotype, comorbidity, serum ALT, weight, age, and sex. Similarly, Akhondi et al. and Bafandeh et al. also concluded that the response to interferon-based treatment was not related to the HCV genotype (23, 24).
On the other hand, in Zeuzem et al. study (6), there was a difference in the response between type 2 and type 3 HCV genotypes so that HCV patients with type 3 genotype needed a longer treatment period with sofosbuvir-ribavirin. SVR12 was seen in 93% of the patients with HCV genotype 2 who were treated for 12 weeks and in 85% of the patients with HCV genotype 3 treated for 24 weeks. In that study, four potential predictors of high SVR rates were mentioned as female sex, lack of liver cirrhosis, lower age, and low viral load at baseline (6). These factors were also found to be effective in Osinusi et al. investigation (20). In the study of Bourliere et al., a lower response rate was observed in patients with liver cirrhosis, higher viral loads, and black race (25). In the study of Charlton et al. the highest response rates for sofosbuvir and ribavirin were observed in patients with HCV genotype 1 and with a previous history of interferon treatment (18). These results are not consistent with the findings of this study because there were no significant differences in the response to treatment based on various factors such as the type of genotype, age and/or cirrhosis of the liver although, in this study, there was not any patient with genotype 2. This discrepancy in results could be due to differences in selected populations and should be confirmed by other studies before consideration.
The other findings of the current study included safety, high compliance rate, and lack of mortality or significant morbidity. In this study, only minor side effects including a headache and fatigue were observed (Figure 1). During the current study, three cases did not complete the course of therapy. Two cirrhotic patients stopped their medication due to liver decompensation while one cirrhotic patient complicated with CVA and excluded from the study. It is not clear that this liver decompensation or CVA is related to therapy with sofosbuvir or some other reasons. In the medical literature, we were unable to find any similar relationship between therapy with direct acting agents (DAA) and liver decompensation and/or CVA.
Ruane et al. found that the 24-week treatment with sofosbuvir + ribavirin is an effective and well-tolerated treatment for patients with Genotype 4 HCV infection. A headache, insomnia, and fatigue were the most common side effects among the patients of that study (17). Their results are consistent with the findings of the current study. In Osinusi et al. study, the combination of sofosbuvir and ribavirin showed a high safety profile and again, the most frequent side effects included a mild-to-moderate headache, anemia, fatigue, and nausea. There were also seven cases of major side effects including anemia, neutropenia, nausea, hypophosphatemia, and gallstones pancreatitis. No patient left untreated due to side effects (20).
In Zeuzem et al. study, no serious side effects were found. However, minor side effects were observed in 86 to 92% of the patients, with a headache, fatigue, and/or itching as the most common ones (6). In a study by Charlton et al., there was no detectable virus resistance during or after the treatment. Fatigue (30%), diarrhea (28%), and headache (25%) were the most common side effects. In addition, 20% of the patients experienced anemia (18).
In general, all studies, in concordance with the current study, demonstrated the safety and high tolerability of the treatment with sofosbuvir. The minor differences in observed side effects are related to the differences in the study populations, the patient's profiles, and the differences in the immune systems of different individuals. The limitations of our study included the small sample size, lack of liver biopsy to determine the exact extent of the disease and its relationship with HCV genotype.
4.1. Conclusion
The findings of this study indicated that the combination of sofosbuvir and ribavirin for 24 weeks, regardless of genotype, underlying disease, and/or previous treatment experience, is an effective therapeutic regimen for HCV patients, with high tolerability and almost negligible side effects. Based on the evolution of new therapeutic regimens and direct-acting agents, it is worthy to design new sofosbuvir-based therapeutic regimens in combination with newer direct-acting agents to achieve even shorter effective protocols.