1. Background
Non-alcoholic fFatty liver disease (NAFLD) is one of the most common diseases in the world that affects most obese, inactive people and those with type 2 diabetes (1). This disease is described by elevated triglyceride levels, liver enzymes, some inflammatory biomarkers, and liver steatosis (2). The incidence rate of this disease in Asia is 12% - 24%. In Iran, the prevalence of NAFLD and liver steatosis varies between 2.9% and 7.1% (3). NAFLD is known as a progressive metabolic disease potentially damaging the liver, which exposes patients to advanced liver failure. Given the multifactorial nature of this disease and the progression of this chronic disease, the results of NAFLD treatment are difficult. According to the relationship between insulin resistance and oxidative stress on liver damage, it can be the basis for fat accumulation and disease progression. Drug therapy, e.g., with metformin, is associated with increased insulin sensitivity (4). In their meta-analyzes, Musso et al. stated that most studies were conducted to reduce insulin resistance and decrease cardiovascular risk factors and that weight loss and lifestyle change could prevent disease progression (5). NAFLD treatment is based on nutritional control, medical treatment, and physical activity (2). Much research has focused on the effects of medicines and non-pharmaceutical interventions on this disease. Although the definitive treatment for this disorder has not yet been found, researchers have proposed lifestyle change and a combination of adequate diet and physical activity to prevent and treat this illness (6).
Over the past decades, physical activity has been a key contributor to the control of NAFLD along with medical therapy. Studies have shown that various exercise protocols are effective in reducing the prevalence and improvement of some metabolic functions of the liver (7). Research has shown that exercise training is one of the effective factors in body composition and active people have a lower percentage of body fat (PBF) than passive people (8). On the other hand, people who do not have the opportunity to attend sports places, as well as those who, despite having exercises, do not see a change in their PBF or have excessive expectations for their body fat, consider the use of fat burning supplements and the use of these compounds is increasingly on the rise; among these supplements is L-carnitine. L-carnitine is an active form of carnitine in the body that is prescribed and consumed orally (9).
Engel et al. mentioned in a study that low levels of L-carnitine in human muscle fibers are in a relationship with obesity and fat stores and they described it as a new syndrome (10). Also, Karlic and Lohninger showed that L-carnitine deficiency could lead to obesity by reducing fat oxidation and accumulation of fatty acids and triglycerides in adipose tissues (11). However, in some studies, the role of this supplement in fat burning and weight loss was not confirmed (12-14). L-carnitine is a quaternary ammonium compound that biosynthesizes lysine and methionine amino acids. This is performed in the liver and kidneys and stored in skeletal muscles, brain, heart, and sperm. Carnitine is essential for the transfer of fatty acids from cytosol to mitochondria (13). In fat metabolism, Acetyl-CoA is bound to carnitine by carnitine acetyltransferase I in the mitochondrial membrane. The carnitine acetyl produced by carnitine-acetylcarnitine translocase is transmitted to the mitochondria and eventually converted to acetyl coenzyme A by acetylcarnitine transferase II in the internal membrane of the mitochondria; thus, carnitine is re-released to the cytosol (15). Several studies have shown that L-carnitine supplementation significantly reduces the percentage of body fat (16). Dayanandan et al. showed that using L-carnitine reduced lipid peroxidation, improved the antioxidant status, and was effective in liver function improvement (9). Also, several studies have shown that L-carnitine supplementation can reduce the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) enzymes (17, 18), which indicates the complementary role of carnitine in hepatic function improvement.
Considering that carnitine plays an important role in the metabolism of fats and lipolysis and because of its availability, low cost, and complications for those who are seeking weight loss, the use of this supplement has found a high prevalence (9). On the other hand, one of the usual recommendations for physical fitness improvement (16) and hepatotoxicity (6) is the exercise.
2. Objectives
The purpose of this study was to compare the effects of exercise and L-carnitine supplementation, alone or in combination, on body composition and ALT/AST liver enzymes in obese men with NAFLD.
3. Methods
3.1. Sample Selection
In the present semi-experimental study, 40 obese men with NAFLD were selected by purposeful sampling. Then, using a random number table, they were assigned to one of four groups includes: (1) resistance training, (2) supplement, (3) combined (exercise- supplement) and (4) control. After a phone call, the sampling was done through targeted sampling from among volunteers who had entered the study. The study design was explained to volunteers in private interviews and face-to-face meetings. Eligible volunteers were selected by a physician after signing consent forms. The study protocol and its ethical considerations were approved by the Abadan Islamic Azad University (grant No. 18144). A researcher-made questionnaire was used to gather demographic data such as age, marital status, education, history of the disease, and physical activity status.
The Inclusion criteria included BMI of 30 - 35 kg/m2, inactive lifestyle (no physical activity in the past six months), liver fat levels of 1 - 3 grade, non-smoking, non-acute cardiovascular disease, respiratory diseases, and musculoskeletal and skeletal disorders. The exclusion criteria included receiving any other intervention except for the intervention intended in this study implemented by the investigator and susceptibility or allergy to L-carnitine.
3.2. Anthropometric Indices
The height of subjects was measured without shoes and with a perfectly smooth and forward-looking body. The height of each person was measured in centimeters using the SECA Stadiometer. Weight (kg) was measured using the SECA scale with minimum possible clothing. To measure the body mass index (kg/m2), weight (kg) was divided by squared height and BMI was calculated using the following formula: BMI = weight (kg)/height2 (m). To estimate the percentage of body fat, the 3-SITE SKINFOLD (chest, Abdominal, and thigh) measurements were performed using the Jackson-Pollack (1978) formula (19, 20), as follows:
Body Density = 1.10938 - (0.0008267 × sum of chest, abdomen, and thigh skinfolds in mm) + (0.0000016 × square of the sum of chest, abdomen, and thigh) - (0.0002574 × age)
Percentage of Body Fat = (4.95/BD – 4.5) × 100
3.3. Biochemical Indices
For liver enzymes, blood samples were taken in the fasting state. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured by photometric assay (Bionik kit, sensivity: 1 unit per liter). To prevent the acute effect of exercise on liver enzymes, the measurements were made one day before the start of the study and two days after the last session of the study.
3.4. Research Plan
At the pretest (one day before the intervention), the measurements were performed following the standard methods. Then, interventional training and consumption of L-carnitine were performed in the target groups. Finally, a posttest was performed two days after the 12-week intervention. The control group did not receive any intervention related to exercise and nutrition by the researcher. The control subjects were asked to inform the researcher if they were doing regular exercises or using non-prescriptive pharmaceutical and non-pharmaceutical supplements and to exit them in research if necessary.
3.5. Complementary Intervention
The subjects in the L-carnitine group and resistance + L-carnitine group consumed 10 mg of L-carnitine supplements per kilogram of body weight with main daily meals (16).
3.6. Exercise Training Protocol
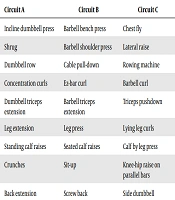
Resistance training (Tables 1 and 2) was done in circles for 12 weeks, three sessions per week, and each exercise session was determined based on previously defined circles and movements. In order to achieve the principle of training variation and reduce the uniformity of exercises, as well as to strengthen the muscles in different angles, the training exercises were performed in a circular manner, and at each training station, a muscle with a similar station was different in other periods, in order to comply with the principle of exercise diversity for the subjects to be studied. Resistance training programs recommend trying to practice all major muscle groups in 8 to 10 exercises per session (21). Accordingly, the resistance training included three circles with nine stations per circle. Maximal strenght was calculated with respect to a maximum frequency using the Brzyski formula and the weight selection was based on the percentage of one repetition maximum (1 RM). However, the repetition of movements in each set was reduced in proportion to the increased intensity of training (practice load).
| Circuit A | Circuit B | Circuit C |
|---|---|---|
| Incline dumbbell press | Barbell bench press | Chest fly |
| Shrug | Barbell shoulder press | Lateral raise |
| Dumbbell row | Cable pull-down | Rowing machine |
| Concentration curls | Ez-bar curl | Barbell curl |
| Dumbbell triceps extension | Barbell triceps extension | Triceps pushdown |
| Leg extension | Leg press | Lying leg curls |
| Standing calf raises | Seated calf raises | Calf by leg press |
| Crunches | Sit-up | Knee-hip raise on parallel bars |
| Back extension | Screw back | Side dumbbell |
| Week | Frequency | Circuit | Repeat | Intensity, 1RM | Rest | Rest Type | ||
|---|---|---|---|---|---|---|---|---|
| Between Movements, s | Between Circuits, min | Between Movements | Between Circuits | |||||
| 1 - 2 | 3 | A B | 15 - 20 | 40 - 50 | 40 - 60 | 3-5 | Inactive, (walking and light activities) | Inactive, (walking and light activities) |
| 3 - 4 | 3 | A B | 15 - 20 | 50 - 60 | 40 - 60 | 3-5 | ||
| 5 - 6 | 3 | A B C | 12 - 15 | 60 - 70 | 40 - 60 | 3-5 | ||
| 7 - 8 | 3 | A B C | 12 - 15 | 60 - 70 | 40 - 60 | 3-5 | ||
| 9 - 10 | 3 | A B C | 10 - 12 | 70 - 80 | 40 - 60 | 3-5 | ||
| 11 - 12 | 3 | A B C | 10 - 12 | 70 - 80 | 40 - 60 | 3-5 | ||
3.7. Statistical Methods
Descriptive statistics, including mean and standard deviation, were used in this study. Paired sample t-test and one-way ANOVA test were used for intra-group comparisons. Furthermore, in the case of a significant one-way ANOVA test, Bonferroni’s post hoc test was used to find the significant pairs. All statistical analyses were performed using SPSS version 22 software at a significance level of P < 0.05.
4. Results
Table 3 summarizes the age, height, weight, body mass index, and fatty liver level of the subjects.
| Groups | Age, y | Height, cm | Weight, kg | BMI, kg/m2 | Fatty Liver, Level |
|---|---|---|---|---|---|
| Exercise | 37.30 ± 2.87 | 167.90 ± 3.81 | 92.45 ± 3.61 | 32.83 ± 1.86 | 2.03 ± 0.23 |
| L-carnitine | 37.80 ± 3.33 | 168.21 ± 5.21 | 94.40 ± 3.83 | 33.45 ± 2.51 | 1.98 ± 0.28 |
| Combined | 38.40 ± 3.17 | 170.52 ± 4.81 | 93.80 ± 3.84 | 32.27 ± 1.05 | 2.11 ± 0.37 |
| Control | 37.10 ± 3.07 | 169.09 ± 4.53 | 92.74 ± 3.43 | 32.33 ± 1.91 | 1.96 ± 0.31 |
Abbreviation: BMI, body mass index.
aValues are expressed as mean ± SD.
Table 4 summarizes the results of the t-test and one-way ANOVA to compare intra-group and inter-group changes in BMI and PBF and shows statistically significant differences in BMI and PBF (P < 0.001). Bonferroni post hoc test showed that there was a significant difference in BMI between the exercise and combined training groups compared to the control and supplementation groups (P < 0.001), but there was no significant difference between the resistance and combination training groups (P = 0.667) and the supplementation and control groups (P = 0.61). In a paired study of changes in PBF, the results showed that there was a significant difference between the exercise and combined training groups compared to supplementation and control groups (P < 0.001), but there was no significant difference between the exercise and combined groups (P = 0.822) and the supplementation and control groups (P = 0.413).
| Variable/Groups | Pretest | Posttest | t | P Value | Percentage Changes | F | P Value |
|---|---|---|---|---|---|---|---|
| BMI, kg/m2 | 18.094 | < 0.001 | |||||
| Exercise | 32.83 ± 1.86 | 32.09 ± 1.79 | 5.772 | < 0.001 | -2.25 | ||
| L-carnitine | 33.45 ± 2.51 | 33.29 ± 2.58 | 0.931 | 0.376 | -0.47 | ||
| Combined | 32.27 ± 1.05 | 31.32 ± 1.10 | 9.029 | < 0.001 | -2.95 | ||
| Control | 32.33 ± 1.91 | 32.68 ± 1.79 | -2.348 | 0.043 | 1.11 | ||
| PBF % | 31.035 | < 0.001 | |||||
| Exercise | 29.86 ± 1.51 | 28.07 ± 1.48 | 7.630 | < 0.001 | -5.98 | ||
| L-carnitine | 30.30 ± 1.35 | 30.26 ± 1.58 | 0.125 | 0.903 | -0.11 | ||
| Combined | 30.21 ± 1.17 | 28.11 ± 1.06 | 9.889 | < 0.001 | -6.91 | ||
| Control | 30.68 ± 1.73 | 31.14 ± 1.58 | -2.187 | 0.056 | 1.56 |
Abbreviations: BMI, body mass index; PBF, percentage of body fat.
Table 5 summarizes the results of t-test and one-way ANOVA to compare the intra-group and inter-group changes in serum AST and ALT enzymes. The results of statistical analysis showed a significant difference in the percentage of changes in AST and ALT (P < 0.001). Bonferroni post hoc test was used to find the location of the difference. The results showed that there was a significant difference between the exercise group, L-carnitine, and combination groups, and the control group (P < 0.001, P = 0.001, and P = 0.003, respectively). There was also a significant difference between the combined and the exercise and supplementation groups (P = 0.001 and P < 0.001, respectively), but there was no significant difference between the exercise and supplementation groups (P = 0.997). There was a significant difference in the ALT changes between the resistance training, L-carnitine, and combination groups, and the control group (P = 0.003, P = 0.002, P = 0.001, and P < 0.001, respectively). There was also a significant difference between the combined group and the exercise and supplementation groups (P = 0.017 and P = 0.010, respectively), but there was no significant difference between the exercise and supplementation groups (P = 0.997).
| Variable/Groups | Pretest | Posttest | t | P Value | Percentage Changes | F | P Value |
|---|---|---|---|---|---|---|---|
| AST, U/L | 24.140 | <0.001 | |||||
| Exercise | 37.50 ± 4.35 | 31.90 ± 3.84 | 6.952 | < 0.001 | -14.26 | ||
| L-carnitine | 32.20 ± 5.75 | 26.60 ± 4.50 | 6.159 | < 0.001 | -17.03 | ||
| Combined | 34.40 ± 4.12 | 23.40 ± 5.50 | 11.524 | < 0.001 | -32.59 | ||
| Control | 29.00 ± 3.03 | 29.30 ± 2.71 | 0.238 | 0.817 | -0.25 | ||
| ALT, U/L | 17.092 | < 0.001 | |||||
| Exercise | 47.20 ± 5.07 | 39.60 ± 4.95 | 7.501 | < 0.001 | -16.04 | ||
| L-carnitine | 43.60 ± 6.06 | 36.10 ± 4.56 | 7.243 | < 0.001 | -16.88 | ||
| Combined | 44.50 ± 4.09 | 31.40 ± 6.26 | 9.189 | < 0.001 | -29.71 | ||
| Control | 38.80 ± 3.97 | 38.50 ± 5.50 | 0.210 | 0.839 | -0.55 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
5. Discussion
In the present study, there were significant decreases in body composition (BMI and PBF) and ALT/AST serum enzymes after resistance training when compared to the control group. According to Nunes et al. (22), after 16 weeks of resistance training, there was a significant decrease in PBF of healthy female volunteers. Moradi Kolardeh et al. (23) also showed a significant decrease in ALT and AST serum enzymes of NAFLD patients after 12 weeks of resistance training. Nikseresht et al. (24) also reported a significant decrease in the BMI and PBF of healthy obese men after 12 weeks of resistance training. Eslami et al. (25) confirmed the role of exercises in reducing BMI and liver fat percentage. Ghahramanloo et al. (26) reported a significant decrease in fat mass of non-trained men after eight weeks of resistance training. But, several studies have reported contradictory results. For example, Mohammad-Rahimi and Attarzadeh-Hosseini (27) did not report a significant difference in ALT and AST serum enzymes after aerobic training in women with type 2 diabetes. The possible reason for differences in the outcome may be the differences in subjects’ characteristics such as sex and diabetes status between Mohammad-Rahimi and Attarzadeh-Hosseini research and the current study. Also, the exercise protocol in the present study included circuit resistance training that was different from the aerobic training in the study by Mohammad-Rahimi and Attarzadeh-Hosseini.
Bacchi et al. (28) reported a significant decrease in BMI and PBF after four months of resistance training and aerobic exercise, but did not report significant differences in the ALT and AST serum enzymes in women with type 2 diabetes. This is while in this study, both body composition and ALT/AST enzymes improved. One of the reasons for the difference in results is the difference in subjects’ characteristics, such as diabetes status, or the differences in the training program, which was a circuit resistance training exercise in the present study rather than classic resistance training in Bacchi et al. research.
One of the most important factors affecting liver hemostasis is the level of physical activity. Longitudinal research suggests that those who are prone to fatty liver, such as obese or diabetic patients, will suffer from liver enzyme disorders in the case of low physical activity (6). Therefore, physical activity can be one of the factors affecting the improvement of liver function. Regarding the association of energy consumption with exercise and physical activity, the exercise is one of the most useful and inexpensive ways to prevent or treat liver problems and lifestyle changes, physical activity, and exercise can reduce weight and improve liver enzymes (6). Generally, the longitudinal and systematic research findings indicate that exercises are effective in improving hepatic steatosis and metabolic abnormalities in NAFLD (29).
The results of this study also indicated the role of circuit resistance exercise as an effective training method for improving body composition and decreasing ALT and AST serum enzymes as the signs of liver function. In the supplement group, after a period of L-carnitine use, the ALT and AST serum enzymes were significantly lower than in the control group. Demiroren et al. (18) reported the protective role of L-carnitine in liver fibrosis in a laboratory study. Malaguarnera et al. (17) also reported a significant reduction in ALT and AST serum enzymes after 24 weeks of L-carnitine supplementation in non-alcoholic steatohepatitis patients. The results of the current study are in line with the findings of this research. However, there was no significant difference in body composition (BMI and PBF) after L-carnitine supplementation between the intervention group and the control group.
However, Haghighi et al. (16) in a study conducted in 2010, after a period of L-carnitine consumption, reported a significant reduction in PBF of middle-aged men that was not consistent with the results of the present study, possibly due to the difference in subjects’ characteristics or other intervening factors, including diet, which was not controlled in our study and it is considered a research constraint. In the combined group, after a resistance training course with L-carnitine, there was a significant decrease in ALT and AST serum enzymes and improvements in body composition (significant decreases in BMI and PBF) when compared to the control group. Haghighi et al. (16) investigated the effect of aerobic exercise with L-carnitine supplementation and showed a significant reduction in the PBF of active overweight middle-aged men, which is consistent with the results of the present study. Several studies have shown that L-carnitine supplementation accelerates fat oxidation in overweight subjects (16, 30, 31). In fact, the regular use of carnitine increases plasma and intracellular concentration of carnitine and results in increased fat oxidation and gradually decreased body fat stores (11, 16). In a study, Lofgren et al. (14) reported no difference between the L-carnitine supplementation group or placebo and aerobic training and high-protein diet intake group in terms of body weight, body massindex, fat percentage, and waist-to-hip ratio. In the present study, L-carnitine supplementation alone did not show a significant difference in body composition, but there was a significant difference between the combination group and the control group. It shows the role of physical activity in improving body composition. Also, in the combination group, the changes in liver enzymes were significantly higher. It seems that resistance training and L-carnitine complementary together have a more consistent effect on improving liver function and these changes are independent of body composition changes. Considering that one of the factors affecting the production of fatty liver is insulin resistance, Malaguarnera et al. (17) reported a reduction in insulin resistance after taking L-carnitine supplementation. It is likely that changes in liver enzymes in L-carnitine supplementation groups are due to a decrease in insulin resistance that was not measured in this study; thus, it is another limitation of our research.
Comparing the methods of intervention on the body composition, the exercise group and combination exercise-supplement group, a significant reduction was observed in BMI and PBF in comparison with the control and L-carnitine supplementation groups. In other words, to improve body composition, L-carnitine supplementation did not have a significant effect and the intervention exercise was needed to do so. Comparing the methods of intervention concerning liver enzymes, although all three interventions were saliently effective, changes in the combination group were significantly higher than those of resistance and L-carnitine complimentary groups, suggesting the interactive role of L-carnitine supplementation and resistance training to improve hepatic enzymes.
Regarding the results, it can be said that both exercise training and L-carnitine supplements are effective in improving liver enzymes in NAFLD patients. Also, the combination of exercise training and L-carnitine supplementation can have more efficacy than any single method. Therefore, in the treatment program for patients with non-fatty liver, special attention should be paid to physical activity and active lifestyle.
5.1. Strengths, Limitations and Suggestions
The present study, due to the low sample size, has constraints in the generalization of its results. Since the present study recruited obese men with NAFLD, we should be cautious about generalizing the results to other populations. In the present study, for the first time, the combination of exercise training and of L-carnitine supplementation was applied to change body composition and liver enzymes in NAFLD patients and the results showed improvements in the symptoms of the disease in all three intervention groups, especially in the combination group of exercise and L-carnitine supplements. Therefore, it can be said that the combination of exercise and nutritional interventions can be effective in the treatment of NAFLD. Regarding the fact that sports exercises include many variables such as exercise volume, exercise intensity, dominant energy system, etc., which can affect the results, it is suggested that further research be done in this regard.