1. Background
Malnutrition is a condition that is caused by a decrease or an increase or imbalance in energy, protein, and other nutrients, which leads to measurable adverse effects on body tissues or shape (size and body composition), performance, and clinical status (1). Malnutrition is classified into two main categories: primary malnutrition, which is caused due to inadequate nutrient intake usually because of economic or cultural poverty, and secondary malnutrition that results from maladaptation, illness, alcoholism, substance abuse, increased excretion and neurological disorders in eating (1). Patients in the intensive care unit (ICU) are not homogeneous and have been admitted due to different illnesses and severities (2, 3). Diseases with critical conditions are typically associated with metabolic stress, in which patients develop systemic inflammatory responses (2). During the ICU treatment period, with the progression of the disease and decreased appetite or inability to swallow, and in some cases, inappropriate gastrointestinal activity and ultimately inadequate food intake, as well as the increased need for food due to body metabolic response to stress, malnutrition can progress (4). As a result, body metabolism increases and if an adequate amount of calorie and proteins are not provided for metabolism timely, increased catabolism leads to a decrease in fat deposits, which causes a reduction in muscle mass (5, 6) and malnutrition of protein and energy, which is a major problem in hypercatabolic patients with high severity of disease in the ICU (7-9). Proper nutrition is one of the care standards in ICU, which partly emphasizes the regular and targeted blood glucose monitoring in patients with critical conditions. Studies have shown that maintaining blood glucose in the range of 80 - 110 mg/dL leads to a 42% reduction of mortality in ICUs (10).
Today, the European Society for Parenteral and Enteral Nutrition (ESPEN) institute is one of the most famous global institutes in collecting nutrition guidelines for different patients with different conditions (11).
The ESPEN is an international community that accelerates the development of clinical guidelines in the field of nutrition (12-14). The accuracy of the published guidelines by ESPEN institute is so widely accepted, and most European countries use it as a clinical guideline (15-19). The nutrition guideline for ICU patients has been compiled by the best researchers of this institute (20).
Although proper nutrition intake is an essential part of the treatment of malnutrition, few studies have considered patients’ intake in ICUs (5). In Iran, there is no comprehensive data on malnutrition rates in hospitalized patients, but in a survey conducted in hospitalized patients in ICUs, it was found that there was a significant difference between the calorie intake and the caloric target in the patients admitted in these wards (5). The incidence of malnutrition in ICU patients is higher than in other patients (5, 6).
2. Objectives
After identifying the guidelines of the ESPEN institute as one of the most comprehensive guidelines for patients admitted to the ICU, this study was conducted to determine the effect of using a nutrition guideline by nurses on the incidence and severity of malnutrition in hospitalized patients in ICU.
3. Methods
This single-blind clinical trial was conducted on all diabetic patients at risk of malnutrition in the ICU of Golestan and Imam Khomeini hospitals of Ahvaz who have been fed by mouth or through the nasogastric or gastrostomy tubes. Patients available who met the inclusion criteria were randomly divided into the intervention and control groups. Inclusion criteria included having chronic diabetes, age of 14 and over, admission to the ICU up to 24 h after admission to the hospital, staying and admission to the ICU for at least 10 days, feeding the patient during 10 days of staying in ICU through oral feeding or the nasogastric or gastrostomy tubes, no hyperparathyroidism, no renal failure, no pheochromocytoma, no use of corticosteroids, no liver disease or severe infection, no injection of protein-containing components, no severe dehydration, and the central venous pressure of less than 2 cmH2O due to false increase in serum pre-albumin. Exclusion criteria included the unwillingness of patients to participate in the study, pregnancy, lactation, smoking, using immunosuppressive drugs, following specific diets, changing the diet or deciding to lose weight, and leaving the ICU department in less than 10 days of admission. The sample size using the formula was determined as 35 subjects that according to the control group, finally, 70 cases were included and randomly divided into two groups of 35 cases as the intervention and control groups. The sample size was calculated based on the results of relevant studies with a 95% confidence interval and 80% power using the following formula:
After determining the two groups, the level of pre-albumin, albumin, and total lymphocyte count was checked for each participant using the nephelometry method, and the results were recorded in the checklist paper, followed by calculating their body mass index (BMI). Then, 2 cc of blood was taken from the patient’s vein and pre-albumin, albumin, and serum lymphocyte count were measured immediately.
After collecting initial information of the patients, the researcher determined the diet and nutrition requirements for each patient in the intervention group using the nutrition guideline and considering the laboratory test results, as well as clinical symptoms, and notified the nurse and the relevant authorities in the kitchen. The researcher checked all patients for new information twice daily.
After 10 days, blood samples were taken from patient and pre-albumin, albumin, and serum lymphocyte count, as well as BMI of the patients, were determined in the same manner and recorded in the checklist. They were also measured for the control group. The data were collected by a questionnaire containing a patient’s demographic information, anthropometric measurements, and type and route of nutrition. The data collection tool was a demographic questionnaire, including demographic characteristics, anthropometric measurements, type of nutrition and feeding routes, as well as the Maastricht index for malnutrition. In this index, according to the albumin, a complete count of lymphocyte, pre-albumin, and ideal patient weight, a score was given to the patient. The values below zero did not indicate malnutrition and the values of equal to zero or more indicate malnutrition in the patient. In other words, the lower the rate on this index represents the better nutritional status of the patient (21).
Maastricht index formula:
MI = 20.68 - (0.24 × serum albumin g/L)) - (19.21 × serum pre-albumin g/L) - (1.86 × TLC L (106 cells/g) - (0.04 Ideal body weight)
To assess the validity and reliability of the Maastricht Index, in a study by Naber et al. (22), aimed at investigating the prevalence of malnutrition in the hospital using the Maastricht index and nutritional risk index, and subjective global assessment indices, and correlating the results of each index with the patient’s therapeutic return and the prevalence of complications, this index was selected to estimate complications in malnutrition patients. Another study reported a sensitivity of 95% and specificity of 94% for the Maastricht index (23).
A meter was used to determine the patients’ height, and to determine the patients’ weight, a digital or analog balance was used.
Ethical considerations of this study included obtaining permission from the Jundishapur University of Medical Sciences officials and coordinating with the Ahvaz Health Network and covered Health centers, and obtaining the approval from the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences. The research process was undertaken after obtaining the written informed consent and all participants were informed that they had the option to withdraw from the study whenever they wish. This study was approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (Ethical code: IR.AJUMS.REC.1396.993) and registered at Iran Clinical Trial Center (no.: IRCT20180314039097N1).
For statistical analysis, SPSS software version 23 was used. Data were reported as mean ± SD. The Kolmogorov-Smirnov test was used to assess the normal distribution of data. To compare the results before and after the intervention in each group, the paired t-test was used, and the independent t-test was applied to compare the results between the two groups. P value of less than 0.05 was considered statistically significant.
4. Results
Of the 75 patients, clinical and laboratory evaluations were completed for 70 patients. Two cases of the control group and one case of the intervention group were excluded from the study because of the need for extra days (one patient suffered from abdominal distension and nil per os (NPO) and two patients required tracheostomy). One patient in the control group and one in the intervention group died. Table 1 shows the demographic characteristics of the studied patients.
| Intervention Group | Control Group | Level of Significance | |
|---|---|---|---|
| Age, y | 70.28 | 66.74 | 0.392 |
| Ideal body weight, kg | 69.51 | 67.69 | 0.280 |
| Primary body mass index, kg/cm2 | 22.04 | 21.80 | 0.804 |
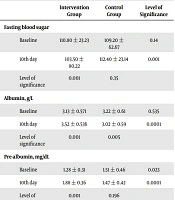
There were no significant differences in age and ideal weight, as two baseline indices, in two groups (P = 0.392 and P = 0.280, respectively). It is worth noting that the ideal weight parameter is calculated by the Maastricht index, and the lack of difference between the two groups in this regard initially eliminates the probability of bias due to its non-difference in the two groups. The amounts of laboratory parameters effective in evaluating in Maastricht method (albumin, pre-albumin, and total lymphocytic counts) are also included in Table 2.
| Intervention Group | Control Group | Level of Significance | |
|---|---|---|---|
| Fasting blood sugar | |||
| Baseline | 110.80 ± 23.23 | 109.20 ± 62.67 | 0.14 |
| 10th day | 103.50 ± 80.22 | 112.40 ± 23.14 | 0.001 |
| Level of significance | 0.001 | 0.35 | |
| Albumin, g/L | |||
| Baseline | 3.13 ± 0.571 | 3.22 ± 0.61 | 0.535 |
| 10th day | 3.52 ± 0.536 | 3.02 ± 0.59 | 0.0001 |
| Level of significance | 0.001 | 0.005 | |
| Pre-albumin, mg/dL | |||
| Baseline | 1.28 ± 0.31 | 1.51 ± 0.46 | 0.023 |
| 10th day | 1.88 ± 0.36 | 1.47 ± 0.42 | 0.0001 |
| Level of significance | 0.001 | 0.196 | |
| Total lymphocytic counts, cell/mm3 | |||
| Baseline | 1638.2 ± 489.5 | 1591.57 ± 505.2 | 0.696 |
| 10th day | 2195.40 ± 500.8 | 1575.23 ± 476.6 | 0.0001 |
| Level of significance | 0.001 | 0.286 |
aValues are expressed as mean ± SD.
Although there was no significant difference in the albumin level at different times between the groups, it was important in determining the overall nutrition of the patient along with other indicators. Using the levels of albumin, pre-albumin, total lymphocytic count, and BMI in the formula, the values of the Maastricht index were determined for each patient. Then, the mean values of the Maastricht index were determined at two time points and in both groups separately. In Table 3, the values of the Maastricht index are shown. The results of t-test showed that according to the Maastricht index values between the two groups after feeding through the two methods, the patients of the intervention group were in a more favorable condition and also considering the normal distribution of the data and using the T-test, it was found that the two groups had a significant difference (P = 0.001).
| Maastricht Index | Intervention Group | Control Group | Level of Significance |
|---|---|---|---|
| Before intervention | 4.89 ± 2.17 | 4.18 ± 2.62 | 0.222 |
| After intervention | 2.02 ± 2.10 | 3.74 ± 2.26 | 0.002 |
| Level of significance | 0.001 | 0.115 |
aValues are expressed as mean ± SD.
5. Discussion
Malnutrition is a growing problem in many hospitalized patients, especially in patients hospitalized in ICUs (24). It is not a new clinical issue but is an ongoing problem that is remained unresolved, especially in patients who are admitted to the ICUs (25). The presence of malnutrition in patients at the time of admission has been reported with less prevalence in advanced countries (England: 20%, Sydney Australia: 6%, and Canadian elderly: 15%) and a higher prevalence in developing countries (more than 40%) (7, 8, 26, 27). Accordingly, although malnutrition can be presented at the time of admission to the hospital, its prevalence in developing countries is higher than in developed countries, and despite the developments in medicine, there has been no significant decrease in recent years (28).
Studies have shown that lack of attention to the nutritional needs of patients in the ICU leads to increased disease severity, hospitalization time, ventilator dependency, and costs. Malnutrition is described as a common problem that is not well recognized by the medical team. In most medical centers in the world, standard solutions are used to feed patients and meet their metabolic needs. Unfortunately, these solutions are still unknown to Iranian medical staff, and their cost is high for the patient due to a lack of insurance coverage. However, it should be considered that the cost of providing nutritional services and food supplements is negligible compared with the costs of the disease.
In a study by Elia et al. (29), it was stated that on average, about 50% of patients take half or less than half of their meals, and these patients are four times more at risk of malnutrition compared with patients who have more than half of their meals. A prospective study considering ICUs in England showed that the maximum calorie given to patients was between 75% and 85% of the prescribed order (30), and in another study, out of 49 patients, it was found that in the long run, only 11 ICU Patients (23%) had a positive calorie intake, and the rest of the patients had less than their required caloric intake (31). In a study, it was found that calories of the solutions used in the ICU are about one-third of the standard values of the ICUs. Based on the calculations, the caloric intake required for these patients is more than 2000 kcal, yet the calorie intake of patients was about 600 kcal a day (32). These findings have led to the belief that lack of calorie intake is a common problem in ICUs. Adequate nutrition has shortened hospitalization time and patients with moderate and severe malnutrition stay for a twice longer duration in hospital and the death rate of these patients was three times more than those who did not have malnutrition (25).
Our results showed that malnutrition levels were homogeneous, and there was no significant difference at the time of admission and after 10 days of hospitalization in the ICU. However, in general, a 53% prevalence of malnutrition means that more than half of the ICU patients suffer from malnutrition, which is a warning alarm for the treatment teams indicating the need to plan and apply strict nutritional policies at the beginning of the patient’s entry to the hospital. A study found that patients with malnutrition had 30% more problems and complications than those who did not have malnutrition (26). In another study in South America, it was revealed that the costs of patients with malnutrition could be increased by up to 300% more than non-malnourished patients (33). On the other hand, malnutrition causes more deaths in high-risk patients, including patients in the ICU and the elderly. Accordingly, recent investigations suggest that proper calorie intake for critically ill patients should be considered as an essential requirement and a base for treatment (30). In a study by Kruizenga et al. (26) on 7606 patients, 12% of the patients had severe malnutrition, 13% had moderate malnutrition, and 75% had good nutrition, and there was a significant negative correlation between malnutrition and BMI. This association was also reported in the study by Goiburu et al. (30).
Increasing malnutrition rates in hospitalized patients can be due to several reasons, such as increased energy, protein, and micronutrient needs after surgery, and failure to properly meet these needs is one of the most important reasons for the incidence and severity of malnutrition. Failure to meet these needs is also due to the factors, such as patient’s lack of appetite, medications, interactions between medicine and food, emotional and psychological needs of the patient leading to the lack of food intake by the patient, secondary infections, secondary increase in the patient’s need, the lack of attention or awareness of some of the authorities responsible for supplying and distributing food or family members to the patient’s actual needs, and the proper ways to provide their nutritional needs in the hospital environment.
Studies have shown that nutrition counseling and the implementation of various nutritional supportive strategies by the nutrition team in the hospital, especially the ICUs, lead to a reduction in the incidence of malnutrition. Of these strategies, the periodic visit of patients by a dietitian, nursing staff education, and monitoring the hospital’s catering system can be mentioned.
A study done by the Department of Nutrition and Diet Therapy at London’s Hammersmith Hospital during three cross-sectional studies in 1998, 2000, and 2003 showed that nutritional counseling and dietary strategies in patients would reduce the incidence of malnutrition and improve weight gain (28). Malnutrition continues to be a serious problem in ICUs and is associated with inappropriate side effects. If the nutritional needs of patients admitted to ICUs are properly addressed, they can be connected to the ventilator shorter with fewer complications and faster recovery and discharge. Malnutrition, especially in ICU, maybe due to delay in the start of nutritional support and the mismatch of nutritional order with the patient’s weight and recent nutritional history and actual calorie intake. It is also difficult to provide enough nutrients to patients in ICUs due to nutritional suppression as a result of physical examinations, medical interventions, and some digestive problems (34). According to the ESPEN Guideline, if the patient admitted to the ICU is unable to have oral nutrition for up to 3 days, enteral nutrition should be started (25).
As previously mentioned, one of the nutritional support strategies for patients admitted to ICU is the active presence of nursing staff in the nutrition team provided with the training needed to work in ICU. The ESPEN standards published in the Journal of Parenteral and Enteral Nutrition on nutritional support, lists a number of nursing duties in this system, including focusing on protection, promotion, and adjustment of nutritional health, the ability to prevent from injuries and diseases associated with nutrition, and reducing the patient’s suffers and pain. Nutrition formulations should be given to the patient carefully according to a nutritionist’s recommendation and the patient’s tolerance. Also, care should be taken in the patient’s position while receiving nutritional support and up to 3 hours later by the nurse, and the patient’s head should be at least 30 degrees lifted. The nurse should ensure that the formulation is healthy and is free of microbial contamination. Also, the enteral formulations should not be stored at room temperature for more than 12 h. The gastric residual volume should be considered by a nurse to evaluate nutritional tolerance and drug and food interactions (23).
5.1. Conclusions
The obtained results showed a significant difference between the level of albumin, pre-albumin, total lymphocytic count, and in general, the severity of malnutrition based on the Maastricht index before and after the use of the guideline by nurses. The prevalence of malnutrition is high in patients admitted to ICU, which is due to poor nutrition in these units. Although nurses play a significant role in feeding patients, their knowledge regarding the nutritional needs of patients is less than needed. Therefore, considering the necessary backgrounds for an improvement in the knowledge of this group of the treatment staff improve the nutritional status, and subsequently accelerate the process of treatment of patients seems necessary. In this context, nutritional guidelines are helpful in accelerating and facilitate this issue.
5.2. Research Limitations
Due to the time limit and the half-life of three weeks of serum albumin, it was not possible to detect changes in albumin.
The time limit and limited location of this study made it impossible to evaluate the incidence of malnutrition in patients.