1. Background
Obesity is a complex condition characterized by excessive accumulation of adipose tissue. Obesity and overweight are currently the biggest public health problem in the world. These phenomena increase the risk of diseases such as metabolic syndrome, cardiovascular diseases, respiratory disorders and cancer (1). Obesity contributes to the disruption of lipid metabolism resulting in increased levels of low-density lipoprotein (LDL) and circulating triglycerides (TG) and decreased levels of high-density lipoprotein (HDL). Therefore, elevated TG level leads to increased levels of triglyceride-rich lipoproteins, thereby increasing the risk of atherosclerosis and hypertension in obese individuals (2). The incidence of cardiovascular deaths is three times higher in obese men and women (3).
The beneficial effects of endurance training in reducing the risk of cardiovascular diseases have been shown in many studies (4). It has been estimated that fat metabolism rate in working muscles during a single bout of aerobic training is 5 to 10 times higher than resting state (5). Endurance training is a good driver for lowering LDL.
LDL normally carries 60% to 80% of plasma cholesterol, and has a high tendency to adhere to the arterial wall causing the smooth muscle cells of the arterial wall to grow beneath the deposition site and absorb fibroblasts in that area (accelerating blood coagulation in that area); and if this happens in coronary arteries that carry blood to the heart tissue, it may block the supply of sufficient oxygen to the heart tissue, leading to infarction or dead tissue in the heart area, which is a very dangerous and fatal complication (6).
Research findings have shown that exercise can lead to increased beneficial blood lipids, that is, HDL-c. Increased HDL-c will prevent cholesterol deposition inside vessels. On the other hand, exercising, especially aerobic exercise, results in higher fat metabolism, resulting in more fat being used for energy. Fringham et al. reported that with each mg increase in HDL, there is a 2% to 3% reduction in the risk of cardiovascular diseases (7). Hypertension is another risk factor for cardiovascular diseases (8). Results of Murphy et al.’s (9) study showed that following four weeks of hiking, systolic blood pressure decreased by 8% and diastolic blood pressure reduced by 2%. In recent years, there has been a growing focus on the role of nutrition in lowering blood pressure and increasing fat metabolism (10). One thing that is recommended for fat metabolism is caffeine, a nutritional factor that may potentially alter lipid profile (11). Caffeine comprises a crystalline, white, and bitter taste substance called 1,3,7-trimethyl xanthine, consisting of carbon, hydrogen, nitrogen and oxygen (12). It is naturally derived from everyday consumables such as tea, cocoa, coffee beans and chocolate, but is mainly derived from a plant called Caffea Arabica. Scientific findings focus on the beneficial effects of caffeine on physiological functions of the body, including increased energy, reaction speed, alertness and vigilance, accuracy and concentration (12). Some research has also investigated the association between caffeine consumption and cardiovascular risk factors; in resting conditions, it has been shown that caffeine elevates blood pressure and systemic resistance (13). There are contradictory reports regarding the effect of caffeine on the lipolysis mechanism; some research has suggested fat breakdown and elevated plasma free fatty acids levels following caffeine consumption (14). On the other hand, Hulston and Jeukendrup (15) reported that consumption of glucose-caffeine solution had no significant effect on increased lipid oxidation. American College of Sports says that the effectiveness mechanism of caffeine on endurance activities is not well understood. At the beginning of endurance training, caffeine intake may occur to save muscle glycogen consumption, but it is not clear whether this leads to higher fat metabolism and muscle utilization (16). Based on the literature, there is no clear-cut perspective on the effect of caffeine consumption on lipid profile and blood pressure; on the other hand, little research has been performed on the impact of caffeine consumption concurrent with aerobic training. Thus, the results of the present study may help to clarify the existing views on caffeine consumption.
2. Objectives
The present research investigates the effect of endurance training along with caffeine consumption on the lipid profile and resting blood pressure in obese men.
3. Methods
3.1. Subjects
This quasi-experimental study with a pretest-posttest design was conducted as a field study. After the call was announced in Gachsaran, from among non-athlete 40 to 50-year-old obese and overweight male volunteers (BMI ≥ 29), smokers and those with a history of heart disease, hypertension, anemia, diabetes and those who consumed over 300 mg daily of caffeine were excluded from the study. Then, 40 individuals who were eligible for the study were selected as subjects. The subjects were then divided into four groups of 10, including: (1) control, (2) caffeine supplementation, (3) aerobic training, and (4) training + caffeine supplementation. All the subjects completed the consent form and the Physical Activities Readiness questionnaire (PAR-Q). The subjects’ body composition measurements were taken. The subjects’ height was measured using a height meter without shoes. Individuals’ weight was taken using Seca digital model (made in Germany) with light clothing, without shoes, and their body mass index (BMI) was calculated by dividing weight in kilograms by the square of height in meters. Waist circumference was measured by a tape measure at the top of the navel and at the waist area and hip circumference at the most prominent part of the pelvis. Waist-to-hip ratio was also calculated. Systolic and diastolic blood pressure was measured using a MDF800 mercury barometer (made in the United States) twice before and after the training program. Blood sampling was performed intravenously in two stages, one day before the first training session and 48 hours after the last training session in the eighth week and after 10 to 12 hours of fasting (between 7:00 AM and 8:00 AM). After completion of blood sampling, the samples were centrifuged at 3,000 rpm for 20 minutes and the serum was separated and stored at -20°C. To measure HDL and LDL levels, the glucose oxidase enzyme method (biochemistry) was used, also TG and CHO were measured by calorimetric enzyme assay using the Selctera XL (Vital Scientific, Netherlands) auto analyzer. Mean resting blood pressure was calculated by the following formula:
3.2. Caffeine Consumption
The training and supplementation period comprised eight weeks. The caffeine supplementation and endurance training + caffeine supplementation groups daily consumed 5 mg/kg of caffeine supplement brand Alhavin Enercaff, approved by the Iranian Ministry of Health, along with 200 mL of water.
3.3. Exercise Training
The aerobic training program consisted of running; in the first and second weeks, the subjects started training for 20 minutes at 60% of maximum heart rate (HRmax); in weeks 3 - 6, they trained at 65% to 75% of maximum heart rate (HRmax) for 35 minutes, and in weeks 6 - 8, they trained at 75% to 80% of maximum heart rate (HRmax) for 35 minutes (17).
3.4. Data Analysis
In this study, the Kolmogorov-Smirnov test was used to investigate the homogeneity of the groups in terms of different variables. Parametric tests were used because there were no significant differences between groups in terms of different factors (P > 0.05). In order to analyze the hypotheses, t-test was used for intra-group comparisons and one-way ANOVA and Tukey’s post hoc tests were used to compare the study groups. All the tests were performed using SPSS software version 24, and P value less than 0.05 was considered significant.
4. Results
The demographic characteristics of the subjects are presented in Table 1. Changes in LDL, HDL, CHO, TG and mean arterial blood pressure before and after the eight weeks of training program in the study groups are presented in Table 2.
| Group | Measurement Time | Variable | |||
|---|---|---|---|---|---|
| Height, cm | Weight, kg | Body Mass Index, kg/m2 | Weight to Hip Ration | ||
| Control | Pre-test | 174.43 ± 10.34 | 94.32 ± 6.64 | 30.75 ± 1.79 | 97.73 ± 6.64 |
| Post-test | 94.77 ± 7.34 | 30.88 ± 1.83 | 98.11 ± 6.99 | ||
| Caffeine | Pre-test | 176.12 ± 7.72 | 95.40 ± 8.76 | 30.81 ± 1.65 | 97.84 ± 7.16 |
| Post-test | 95.20 ± 6.31 | 30.78 ± 1.59 | 97.74 ± 5.87 | ||
| Aerobic | Pre-test | 177.28 ± 8.72 | 94.51 ± 5.97 | 30.24 ± 1.65 | 96.13 ± 6.32 |
| Post-test | 90.91b ± 4.88 | 29.08b ± 1.58 | 92.88b ± 5.34 | ||
| Caffeine + Aerobic | Pre-test | 177.44 ± 7.81 | 95.13 ± 7.43 | 30.09 ± 1.29 | 97.67 ± 7.98 |
| Post-test | 91.85b ± 5.86 | 29.34b ± 1.42 | 94.23b ± 6.59 | ||
aValues are expressed as mean ± SD.
bStatistically significant difference at P < 0.05.
| Variable | Time | Group | |||
|---|---|---|---|---|---|
| Control | Caffeine | Aerobic | Caffeine + Aerobic | ||
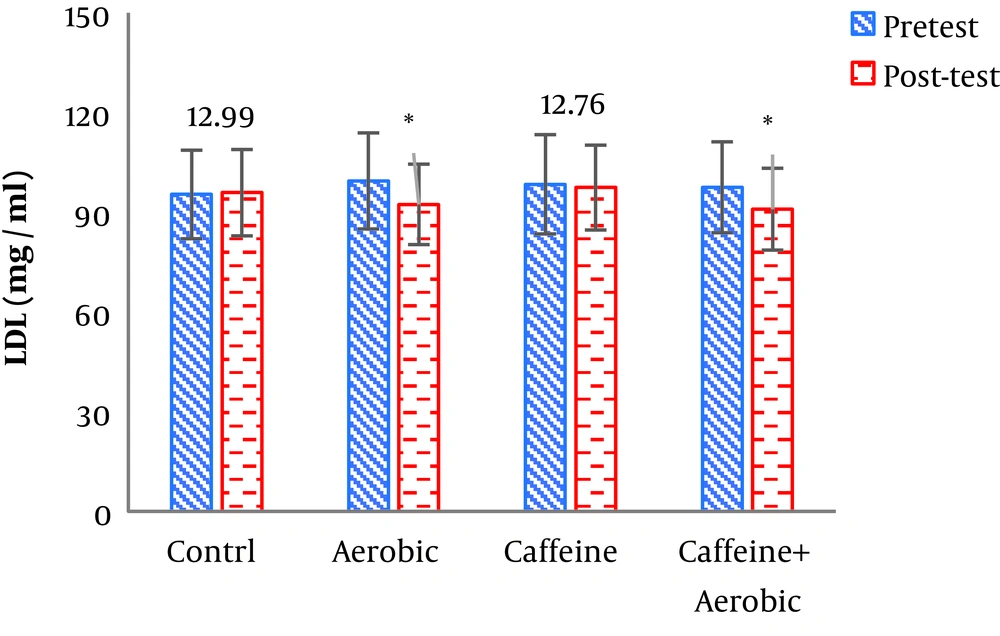
| Low-density lipoprotein, mg/dL | Pre-test | 95.33 ± 13.32 | 98.40 ± 14.94 | 99.41 ± 14.45 | 97.51 ± 13.68 |
| Post-test | 95.88 ± 12.99 | 97.42 ± 12.76 | 92.35 ± 12.1 | 90.9 ± 2.32 | |
| P value | 0.463 | 0.132 | 0.001b | 0.001b | |
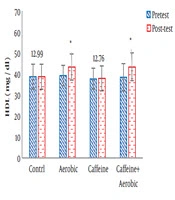
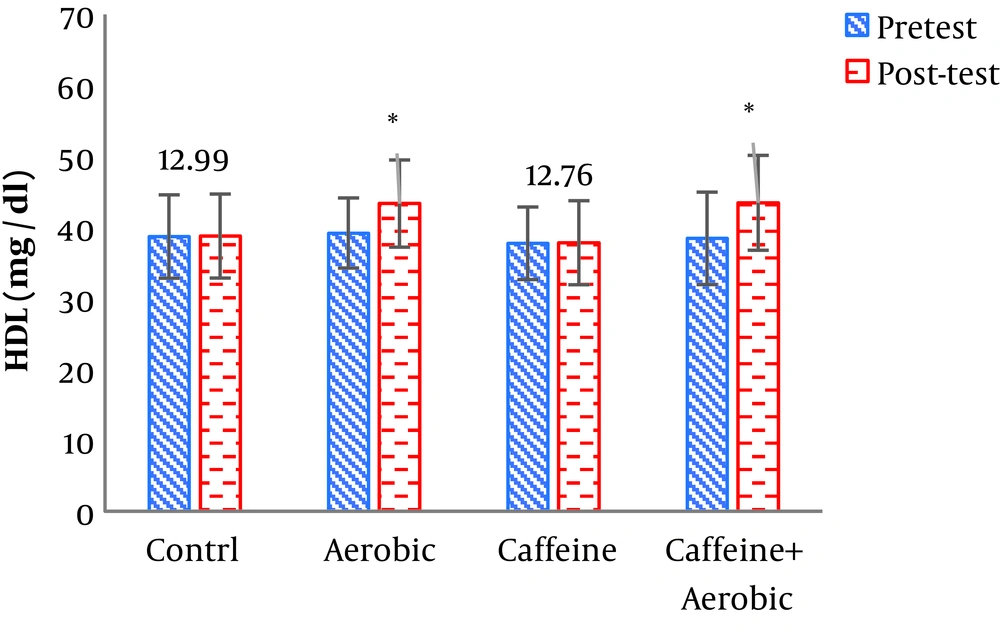
| High-density lipoprotein, mg/dL | Pre-test | 38.81 ± ± 5.87 | 37.76 ± 5.11 | 39.21 ± 4.94 | 38.45 ± 6.51 |
| Post-test | 38.78 ± 5.92 | 37.84 ± 5.79 | 43.55 ± 6.14 | 43.46 ± 6.69 | |
| P value | 0.678 | 0.520 | 0.026b | 0.005b | |
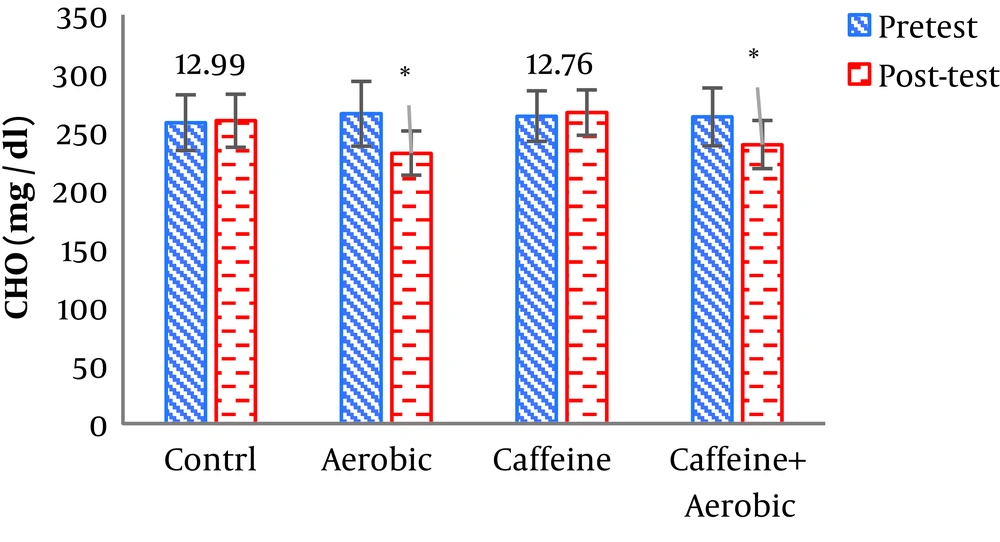
| Cholesterol, mg/dL | Pre-test | 257.65 ± 23.84 | 263.20 ± 21.62 | 265.17 ± 29.76 | 262.54 ± 24.76 |
| Post-test | 259.24 ± 22.70 | 266.22 ± 19.43 | 231.55 ± 18.93 | 238.87 ± 20.61 | |
| P value | 0.319 | 0.204 | 0.001b | 0.002b | |
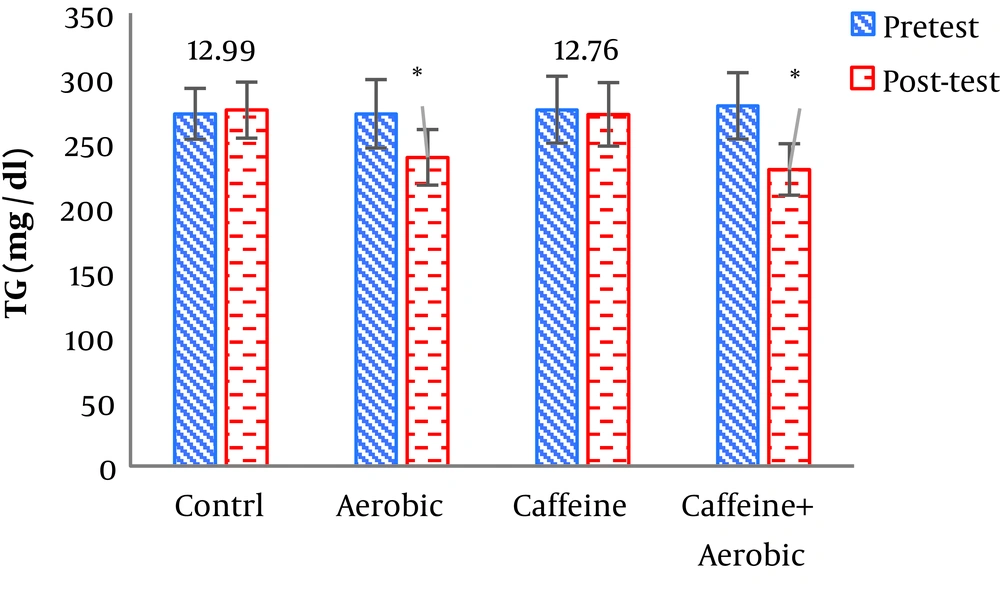
| Triglycerides, mg/dL | Pre-test | 272.19 ± 19.64 | 275.34 ± 25.84 | 272.04 ± 26.42 | 278.32 ± 25.62 |
| Post-test | 274.99 ± 21.69 | 271.74 ± 24.53 | 238.31 ± 21.48 | 229.13 ± 19.88 | |
| P value | 0.843 | 0.472 | 0.002b | 0.001b | |
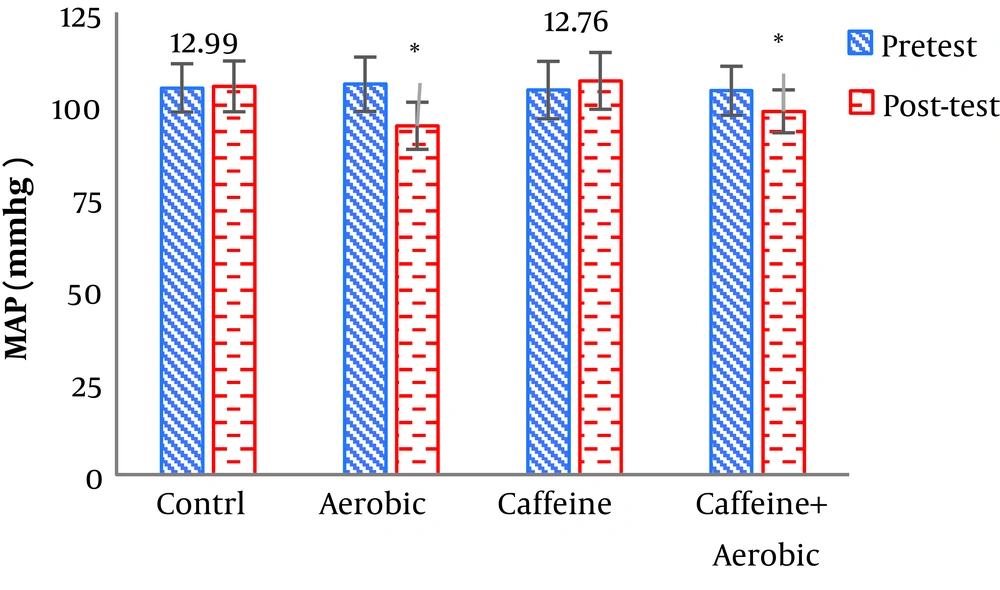
| MAP, mg | Pre-test | 104.56 ± 6.54 | 103.98 ± 7.76 | 105.55 ± 7.34 | 103.79 ± 6.63 |
| Post-test | 104.99 ± 6.87 | 106.45 ± 7.87 | 94.32 ± 6.67 | 98.24 ± 5.80 | |
| P value | 0.352 | 0.201 | 0.001b | 0.006b | |
aValues are expressed as mean ± SD.
bStatistically significant difference at P < 0.05.
The results of dependent sample t-test showed that eight weeks of training along with caffeine supplementation had a significant effect on LDL (P = 0.001), HDL (P = 0.005), CHO (P = 0.002), TG (P = 0.001) and mean arterial blood pressure (P = 0.002). Also, the results of dependent sample t-test in the aerobic training group showed a significant effect on LDL (P = 0.001), HDL (P = 0.026), CHO (P = 0.001), TG (P = 0.002) and mean arterial blood pressure (P = 0.001). In addition, daily supplementation of 400 mg caffeine over two months had no effect on LDL (P = 0.132), HDL (P = 0.520), CHO (P = 0.204), TG (P = 0.472) and mean arterial blood pressure (P = 0.101; Table 2).
The results of one-way ANOVA in Table 3 show significant differences in LDL (P = 0.001), HDL (P = 0.001), CHO (P = 0.014), TG (P = 0.001) and mean arterial blood pressure (P = 0.001) (Table 3). Also, the results of Tukey’s post hoc test in Figures 1-5 indicate that the aerobic training group showed significant differences in LDL (P = 0.003), HDL (P = 0.014), CHO (P = 0.001), TG (P = 0.001) and mean arterial blood pressure (P = 0.001) compared to the control group. Besides, LDL (P = 0.001), HDL (P = 0.002), CHO (P = 0.001), TG (P = 0.001) and mean arterial blood pressure (P = 0.003) were significantly different from the control group. Besides, eight weeks of caffeine supplementation had no significant effect on LDL (P = 0.940), HDL (P = 0.952), CHO (P = 0.998), TG (P = 0.127) and mean arterial blood pressure (P = 0.971) compared to the control group (Figures 1-5).
| Variable | Mean Square | F | Sig. |
|---|---|---|---|
| Low-density lipoprotein, mg/dL | 0.001a | ||
| Between groups | 100.358 | 8.113 | |
| Within groups | 12.369 | ||
| High-density lipoprotein, mg/dL | 0.001a | ||
| Between groups | 36.333 | 7.814 | |
| Within groups | 4.650 | ||
| Cholesterol, mg/dL | 0.014a | ||
| Between groups | 830.567 | 4.084 | |
| Within groups | 203.383 | ||
| Triglycerides, mg/dL | 0.001a | ||
| Between groups | 2975.667 | 117.538 | |
| Within groups | 25.317 | ||
| Mean arterial pressure, mmHg | 0.001a | ||
| Between groups | 464.492 | 16.097 | |
| Within groups | 28.856 |
aStatistically significant difference at P < 0.05
5. Discussion
In the present study, after eight weeks of aerobic training and caffeine supplementation in two groups (caffeine supplement + aerobic training, aerobic training), body weight, BMI, and waist to hip ratio (WHR) were significantly decreased compared to the control group. The results of the study also showed that aerobic training + caffeine supplementation and aerobic training alone significantly decreased serum LDL, CHO and TG levels in the post-test versus pre-test compared to the control and caffeine supplementation groups. Also, serum HDL levels were significantly higher in the post-test than in the pre-test and in comparison with the control and caffeine supplementation groups.
Regular participation in physical activity caused significant changes in LDL, CHO, TG, and HDL levels of the subjects. These results are in agreement with the results of Mukhopadhyay (18), but are in disagreement with the results of Mestek et al. (19). Probably the reason for disagreement in results is related to the subjects, the duration and intensity of aerobic training, or the sampling time. Many studies have shown that different types of exercise training affect blood lipids. O’Donovan et al. (20) in their study showed that exercise activity increased HDL in the experimental group. Exercise training activates lecithin cholesterol acetyltransferase (LCAT), leading to nourishing HDL particles.
Another possible cause of increased HDL is elevated HDL production by the liver following LPL enzyme activity and through plasma triglyceride hydrolysis, as the most important factor in altering HDL concentration (21). Various studies have acknowledged the beneficial effects of aerobic training on reducing LDL (21). Regarding the consumption of lipid as a fuel during both exercise and restoration, it seems that exercise activity is one of the factors reducing LDL. Performing exercise increases the amount of lipoprotein type A and elevates the lipase enzyme level. LPL also catabolizes the lipid portion of LDL, which is therefore expected to decrease in the blood (22).
In the present study, following eight weeks of aerobic training, triglyceride and cholesterol significantly decreased. Changes in triglyceride and cholesterol could be attributed to the lipase response to exercise training. Lipoprotein lipase is one of the regulating lipoproteins and triglyceride breakdown enzymes found in triglyceride-rich lipoproteins. Also. In the present study, the combination of eight weeks of aerobic training and caffeine supplementation improved LDL, CHO, TG, and HDL. Unfortunately, few studies have investigated the simultaneous effects of aerobic training and caffeine supplementation for a long period, and most research has focused on the impact of caffeine intake on athletic performance and athletes’ reaction speed. Caffeine binds to adenosine receptors in the central nervous system and adipose tissue, increasing intracellular cyclic AMP concentration. This enhances alertness, concentration and exhilaration and improves the recall of motor units; also, an increase in cyclic AMP concentration in the adipose tissue, elevates lipolysis. As a result, caffeine saves muscle glycogen consumption during long-term exercise and increases the demand for free fatty acids and their use by active muscles (23). Consistent with the results of the present study, Gonzalez and Stevenson (24) concluded that the effectiveness of caffeine on the lipid oxidation was likely to be significant and effective during low- and moderate-intensity training and it would be blocked during high-intensity training.
Another study found that eight weeks of daily caffeine consumption had no significant effect on LDL, CHO, TG, or HDL. In line with the results of the present study, Graham et al. (25) reported that caffeine consumption had no effects on lipids and carbohydrates metabolism. Some previous studies have suggested that caffeine supplementation may produce some hormonal and enzymatic changes. For instance, it has been pointed out that caffeine consumption inhibits phosphodiesterase, thereby allowing HSL to be more active due to cAMP staying active (15). In addition, the positive effect of caffeine on plasma epinephrine elevation has been observed in several studies (26).
As noted, the present findings reject some of the previous findings on the effect of caffeine consumption on FFA increase (possibly through increased lipases or altered hormone involved in lipolysis). Some of the inconsistencies are due to differences in the dose of caffeine consumed. Some researchers have stated that phosphodiesterase inhibition occurs only at high concentrations (26). In Graham et al. (25) and Sprite study, which compared different doses of 3,6, and 9 mg/kg of body weight, only at the dose of 9 mg increase in glycerol and fatty acid levels was observed. Thus, based on the findings from present and previous studies it can be suggested that caffeine consumption at the recommended doses does not affect the metabolism of caffeine in normal subjects. In other words, consumption of 5 mg/kg of caffeine does not affect lipids metabolism. Other results showed that eight weeks of caffeine supplementation did not cause a significant difference in mean arterial blood pressure compared to the control group. Bruce et al. (27) reported that 500 mg of caffeine had no effect on blood pressure. Blinks et al. (28) concluded that pre-exercise caffeine consumption may elevate blood pressure during training, but ultimately may not raise blood pressure above its normal level during training. The mechanism of action in active and inactive individuals can vary. The findings of the mentioned research were consistent with the present results. In a study with contradictory results to this finding, Ebrahimi et al. (29) showed that consumption of 5 mg/kg of caffeine increased resting systolic and diastolic blood pressure in obese and lean subjects. Other results showed a significant decrease in mean arterial blood pressure in the aerobic training group and the caffeine supplementation + training group compared to the control group, which is in line with the results of Ciolac et al. (30), and inconsistent with the results of Gaeini et al.’s (31) research. According to animal and human studies, a decrease in the sympathetic activity occurs after exercise activity (32). Changes in the vascular reactivity are associated with a decrease in sympathetic conductance for vascular resistance and release of localized dilators (such as nitric acid) due to muscle contraction and increased blood flow to the muscle. After exercise, vascular reactivity to alpha-adrenergic stimulation decreases (33). Topical release of nitric oxide, prostaglandins, adenosine increases during exercise, which facilitates peripheral vasodilation after exercise (34).
5.1. Conclusions
The present study investigated the effect of eight weeks of aerobic training and caffeine supplementation on lipid profile and resting blood pressure in overweight and obese men. According to the present findings, eight weeks of moderate-intensity aerobic training can improve lipid profile and lower mean arterial blood pressure in overweight and obese men. On the other hand, daily consumption of caffeine at a dose of 5 mg/kg for two months in overweight and obese men had no significant effect on their lipid profile and mean arterial blood pressure. As none of the indices associated with lipid profile and mean arterial blood pressure were affected by the dose of caffeine consumption, the present findings do not confirm the effectiveness of caffeine consumption (at least at the dose of 5 mg/kg) in overweight and obese individuals. Overall, by summarizing the present findings and previous research, it can be concluded that the alleged mechanism of caffeine consumption at a dose of 5 mg/kg of body weight (increased lipid motility and increased lipolysis) is questionable, or may only exist at high doses, which can be demonstrated by further studies with larger sample sizes and by careful examination of different doses. Nevertheless, the limitations of the study included the individual differences of the subjects and non-generalizability of the results to the whole community; also, in this study only obese men were selected as research participants, which could prevent the generalizability of the results to obese women.