1. Background
During the last decades, advances in mesenchymal stem cells (MSCs) isolation, expansion, and banking techniques have led to the improvement of the efficiency of cell-based therapies and tissue reconstruction outcome (1). Among various stem cell types, MSCs are intensively applied as therapeutic agents to restore tissue function (2). These cells have the potential to accelerate the healing of mesenchymal origin tissues (3, 4). By using both paracrine and juxtacrine interactions, MSCs can protect the hematopoietic stem/progenitor cells and increase their bioactivities in the bone marrow niche (5, 6). The most available tissues for the isolation of MSCs are samples from bone marrow, umbilical cord blood, and amniotic fluid (7, 8). Owing to these drawbacks and bottlenecks, the establishment of suitable protocols for prolonged cell storage contributes to the on-demand access of patients to distinct cell types. In this regard, many attempts have been collected to increase cell survival after being preserved at deep cryogenic temperature (-196°C) for a long period (9). In many protocols, one or more cryoprotectants are mixed with the basal medium to limit cell damage correlated with intracellular ice formation (10).
Tissue engineering technology provides a platform for the banking system to afford on-demand tissue and cell-based products. Efforts have been made for cell cryopreservation using natural and synthetic polymers (11). Alginate is widely used for the fabrication of 2D and 3D-cellularized scaffolds and microcapsules (12, 13). Microencapsulation is an emerging technology for cell delivery into the target tissues 1. In this technique, cells from various sources are entrapped in the biocompatible matrix, such as alginate surrounded by a semipermeable membrane (14). It showed that cell encapsulation with alginate protected them from different mechanical forces and immune system response while maintaining bidirectional diffusion of molecules (15).
To date, different experiments were performed for the cryopreservation of encapsulated MSCs or mature adult cells by using alginate alone or in combination with various components (16, 17). It was demonstrated that deep freezing of encapsulated human MSCs with 10% dimethyl sulfoxide (DMSO) as cryoprotectants protected cells during freezing/thawing procedure while maintaining metabolic activity and viability compared to the cryopreserved free MSCs (18). Despite the advantages of cell freezing with alginate capsules, the lack of appropriate motifs with a net negative charge necessitates the use of extracellular matrix (ECM) substrates to circumvent these pitfalls (19). In line with this statement, the slow cryopreservation of encapsulated rat pheochromocytoma cell line, PC12, and rat neurospheres with the combined regime of alginate and poly-L-lysine showed better cell viability and a less fragmentation rate (20, 21). Therefore, it seems that the addition of the natural ECM component could improve, but if done not completely, the cell survival rate decreases after freezing/thawing procedure.
To the best of our knowledge, there is little knowledge related to the freezing of encapsulated MSCs with alginate-gelatin capsules. It was demonstrated that gelatin is produced by the denaturation of type I collagen contains motifs that are essential for the bioactivity and dynamic growth of cells (22, 23).
2. Objectives
In the current experiment, we aimed to address the protective effect of alginate-gelatin microcapsules on human MSCs after rapid cryopreservation. We hypothesized that microencapsulation of MSCs could decrease the detrimental effect of freezing/thawing procedure.
3. Methods
3.1. Cell Culture
Human MSC cell line was purchased from the Iranian Biological Research Center (Tehran, Iran). The MSCs were cultured under a standard condition at 37°C with 5% - 10% CO2 and 95% humidity. We incubated the cells in Dulbecco’s Modified Eagle Medium-Low glucose (DMEM/LG; Gibco) with 10% fetal bovine serum (FBS; Gibco), and 1% Pen-Strep (Gibco) and the medium was replenished every 3 - 4 days. Cells at passages 3 - 6 were used to perform the analyses. Moreover, a 0.25% trypsin-EDTA solution (Gibco) was used to detach and transfer the cells during subculture procedure.
3.2. Microencapsulation
An electrostatic method was used to yield encapsulated MSCs by using the mixture of 1% alginic acid sodium (cat no.: 180947; Sigma) and 2% gelatin (cat no.: 1288485; Sigma). To encapsulate cells, 1 mL of alginate-gelatin was mixed with 1 × 106 MSCs and passed through a 25 G needle syringe with a flow rate adjusted to 0.2 mL/min and connected to an 8 kV Electrolysis device (FnM, Hu35p oc, Nigh voltage power supply). Droplets were poured in a solution, containing 1% CaCl2 (Merck) solution stirring at room temperature (13).
3.3. Cell Freezing and Storage
To analyze the protective effect of encapsulation on cell survival rate subjected to -196°C, we divided cells into two different groups, including the control and encapsulated cells. An equal number of cells from each group (1 × 106 MSCs) was mixed with 1 ml cryopreservation medium consisting 20% v/v FBS, 10% DMSO (Merck) and maintained in liquid nitrogen for seven days. Thereafter, MSCs from each group were thawed and subjected to various analyses. To investigate the dynamics of encapsulated cells, MSCs were released by using a 0.01 M sodium citrate solution. Microspheres were incubated with sodium citrate solution until alginate-gelatin shell slowly dissolved away.
3.4. MTT Assay
We performed MTT assay to compare the survival rate of encapsulated MSCs with the control. The basis of the MTT assay is the breakdown of Tetrazolium yellow salt by succinate dehydrogenase and the production of purple crystalline (24). At, respective time point, a number of 10,000 MSCs was transferred into each well, 100 µL MTT solutions from stock (dilution: 4 mg/mL, cat no.: M5655; Sigma) added and incubated at 37°C for 3 - 4 hours. Subsequently, 100 µL of DMSO solution was added to dissolve formazan crystals. The purple-blue color density was measured by a spectrophotometer at 570 nm as a reference wavelength. Cell survival was considered the percentage of the control group (13).
3.5. Analyzing of Growth Dynamic by Cell Cycle assay
At respective time points, cells from each group were incubated with 100 µL of propidium iodide (PI; eBioscience; dilution: 1 µg/mL) for 5 min and washed three times with PBS. Cells were analyzed by the BD FACSCalibur system and FlowJo software version 7.6.1. The percent of cells at phase G0/G1, S, and G2/M were calculated and compared to the control cells (25).
3.6. Flow Cytometry Analysis
To analyze the protective role of alginate-gelatin encapsulation on human MSCs, annexin-V/PI analysis was performed by using a flow cytometry assay. After completion of the treatment protocol, cells were incubated in 100 µL PBS containing 2.5 µL FITC-conjugated annexin-V antibody (eBioscience) for 20 min at 4°C and washed twice with PBS. Thereafter, cells were treated with 2.5 µL PI (1 µg/mL; eBioscience) for 5 min at room temperature and were analyzed by the BD FACSCalibur system and FlowJo software version 7.6.1 (26).
4. Results
4.1. Cell Morphology
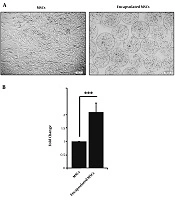
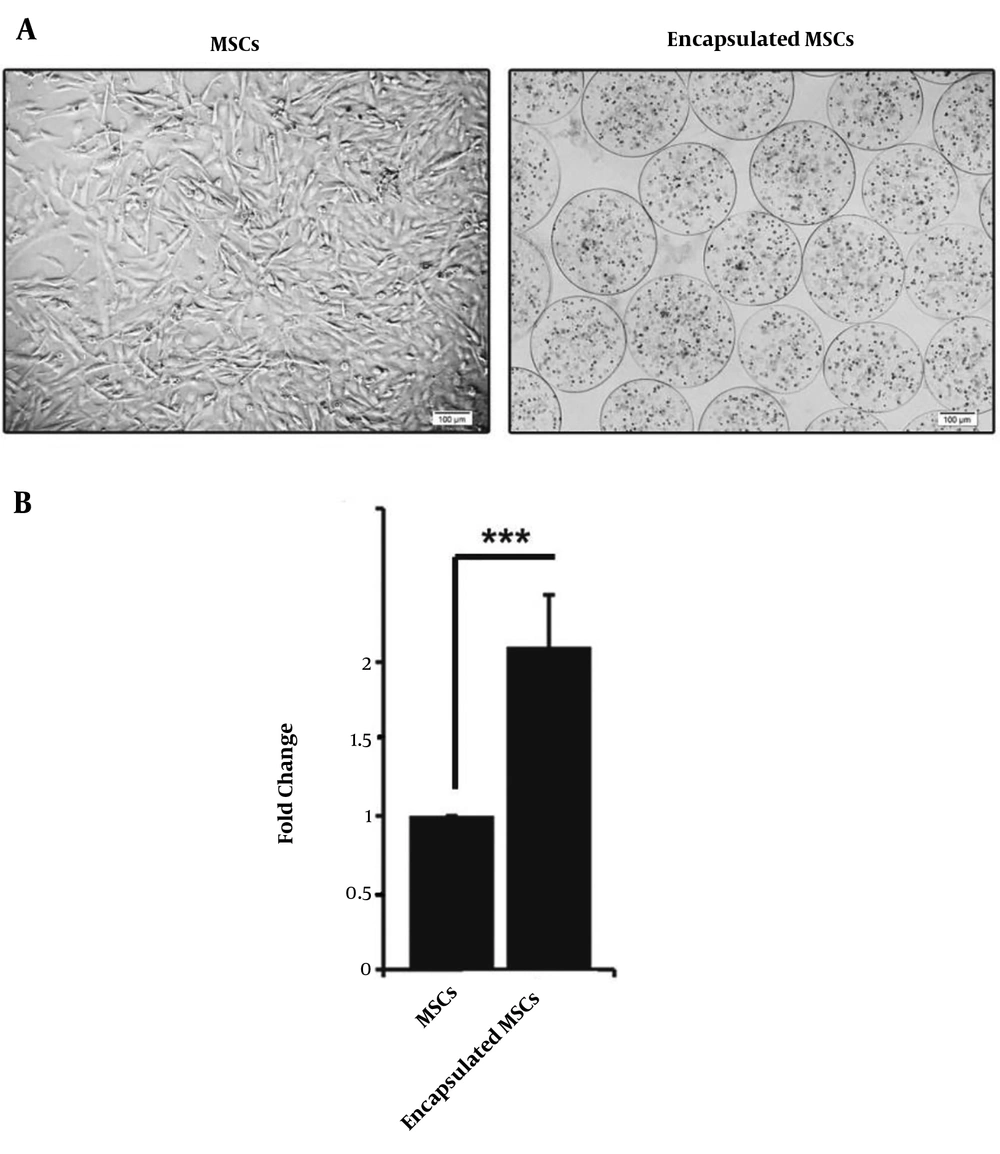
Bright-field microscopy analysis of MSCs showed a difference in cell morphology from both groups (Figure 1A). According to previously published data, MSCs tended to gain spindle-like morphology after expansion on the plastic surface. This cell shape is indicative of MSCs. Based on our results; the mean diameter size of microspheres was 185.03 ± 7.21 µm. We found that MSCs became round-shape inside alginate-gelatin microspheres (Figure 1B).
A, Microscopic bright field monitoring of the control MSCs and encapsulated MSCs cultured for 3 days; B, MSCs tended to acquire fibroblast-like morphology, while encapsulation of these cells with alginate-gelatin provided a round shape and uniform distribution of the cells inside microspheres. MTT analysis was performed to detect cell viability after 7-day cryopreservation in liquid nitrogen. Data showed a significant increase in cell viability compared to the control non-capsulated cells (P < 0.05). Student t-test. ***, P < 0.001.
4.2. Alginate-Gelatin Microspheres Protected MSCs Injury During Cryopreservation
The kinetic growth of MSCs was examined after rapid cryopreservation in control and encapsulated cells (Figure 1). MTT analysis revealed the higher cell survival rate compared to the non-capsulated control cells. Based on data, an approximately 2-fold increase in cell viability was seen in MSC inside alginate-gelatin microspheres. These data show that encapsulation is a promising biological carrier for cell preservation during the freezing/thawing procedure.
4.3. Encapsulation Decreased the Number of Apoptotic and Apoptotic MSCs
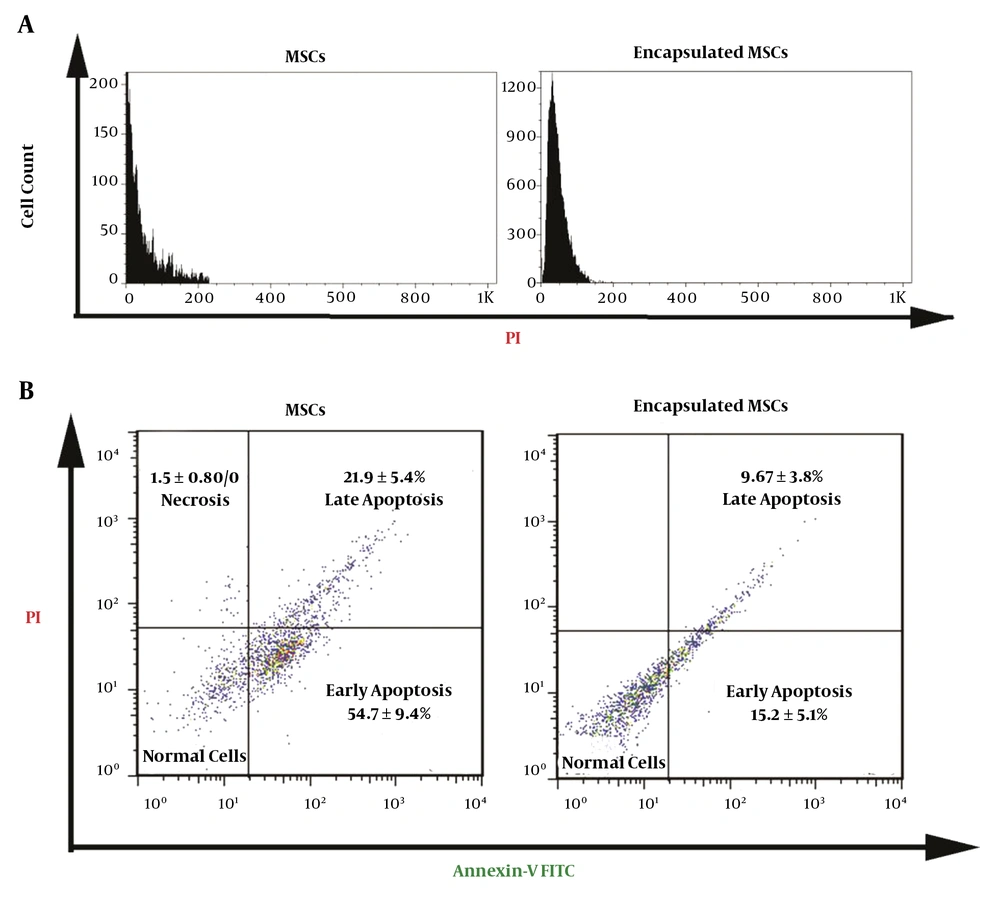
As expected, cell cycle analysis confirmed the lack of cell percentage in S and G2/M phases, showing the inhibition of cell growth and proliferation after being exposed to rapid cryopreservation (Figure 2A). Based on the data, near to the whole cell population belonged to the G0/G1 phase. We performed flow cytometry analysis to explore the potency of microencapsulation on cell mortality. The flow cytometry results revealed that the MSCs encapsulation with alginate-gelatin microspheres decreased the initiation of early and late apoptotic changes, and the number of necrotic cells reached to near-to-zero level while necrosis rate was 1.5% in control. We found ~2.3-fold decrease from 21.9% to 9.67% in the proportion of late apoptotic cells from the encapsulated group. It seems that alginate-gelatin microspheres were able to diminish the onset of early apoptosis in cells during freezing/thawing procedure (54.7% versus 15.2%). The low rate of necrotic cells seen in the current experiment may relate to the sensitivity of these cells to lysis during the preparation for flow cytometry analysis. These data showed the superior effect of the alginate-gelatin microsphere on the inhibition of cell death peculiarly apoptosis during the rapid cryopreservation.
A, Cell cycle analysis confirmed the lack of cells in S and G2/M phases, showing the inhibition of cell growth and proliferation in control and encapsulated MSCs after being exposed to rapid cryopreservation; B, Flow cytometry analysis showed that non-capsulated MSCs showed early and late apoptotic changes compared to MSCs inside the alginate-gelatin microspheres. These data showed that the encapsulation of MSCs could decrease cell apoptosis during deep cryofreezing.
5. Discussion
Mesenchymal stem cells (MSCs) are at the center of attention in tissue repair and regeneration because of distinct multipotentiality and trans-differentiation capacity (27). Since a large number of MSCs are required in the clinic for the synthesis of biological products, the optimization of banking techniques to reach therapeutic cell doses seems essential (28). In this regard, cryopreservation provides short- and long-term storage of MSCs (29). Despite the beneficial effects of cryopreservation, both slow freezing and verification have drawbacks and limitations, leading to cell injury. The formation of ice crystals is often inevitable, and some cryoprotectants are toxic to cells during freezing/thawing procedure. Therefore, cryoprotectant removal seems vital to provide cell viability during thawing and warming to natural temperature (30, 31).
In some cases, the developed cryopreservation recipe for specific cell type is not applicable to the other cells (30, 31). Owing to these drawbacks and bottlenecks, the establishment of suitable protocols for prolonged cell storage contributes to the on-demand access of patients to distinct cell types. To circumvent these limitations, effects have focused on using cryoprotectants in combination with natural polymers such as alginate (1). Microencapsulation is a novel technology for cell delivery into the target tissues and cryopreservation (1).
In the current experiment, we examined the beneficial effects of alginate-gelatin encapsulation on human MSCs’ rapid cryopreservation. Our data confirmed a higher survival rate in encapsulated MSCs compared to the non-capsulated MSCs. Flow cytometry analysis showed the protective role of alginate-gelatin microspheres on the death of MSCs by reducing cell apoptosis after the freeze-thaw procedure. Consistent with our data, Katsen-Globa and co-workers claimed that alginate-gelatin cryogel scaffolds could preserve MSCs stemness and viability (32). It seems that the modulation of mechanical strains and the ability of alginate to absorb water and prevent the formation of ice crystals could decrease cell insult during cryopreservation (33, 34). Wang and colleagues showed that rat adipose-derived stem cells acquire the ability to proliferate in alginate-gelatin hydrogel immediately post-thawing (35). Although the use of alginate-based hydrogels has numerous advantages to protect cells during the cooling/thawing process, it was shown that the addition of gelatin to alginate backbone increased the incorporation of cryoprotectants to the hydrogel. These help to reduce ice crystal formation and facilitate the equilibration of osmotic differences, thereby diminishing cell damage and cytotoxic effects (35).
Establishing novel approaches with the ability to reduce cell damage during the freeze-thaw procedure seems essential. The results of the current experiment showed that alginate-gelatin microspheres, as a 3D scaffold, can reduce cell loss rate and decrease cytotoxic effects of cryoprotectants during cooling/thawing procedure.