1. Background
Research on ionizing radiation is on the rise in the world due to its pharmaceutical, agricultural, and industrial applications (1).
The gamma-ray is the physical process of heat treatment that has proven to be more economical and effective than other ionizing radiation because of their power of penetration, improving the storage lifetime of fruit and vegetables and increasing their bioactive components. Medicinal plants are susceptible to contamination by pathogenic microorganisms during picking, processing, and packing (2). Previous studies have revealed that low doses of gamma-ray with a 60Co source can increase secondary metabolites and inhibit the growth of undesirable microbes (3). There is growing research on medicinal plants as a potential source of secondary metabolites. There are several bodies of evidence that medicinal plants can be used for the treatment of a wide variety of diseases in many countries with lower costs and fewer side effects (4). Phenolic compounds are the most important group of secondary metabolites in medicinal plants (5, 6).
Hepatotoxicity is the most serious health problem worldwide that is caused due to drugs, xenobiotics, and oxidative stress (7). Carbon tetrachloride (CCl4) is a hepatotoxic chemical agent widely used to induce liver damage in animal models. This compound is metabolized into highly reactive species known as ROS, which can attack macromolecules such as proteins, polysaccharides, and DNA. Therefore, the consequent changes cause chronic liver injuries.
Chicory (Cichorium intybus L.) belongs to the Asteraceae family and has been used for the treatment of gastrointestinal and inflammatory disorders. Several biological properties have been reported for chicory root extract such as anti-microbial, anti-hyperglycemias, immunostimulant, antitoxic, anti-inflammatory, and anti-cancer activities (8). The chicory root extract has been shown to possess potential hepatoprotective properties against CCl4 and paracetamol-induced toxicity (9, 10). Cichorium intybus inhibits free radical-mediated injury due to its hepatoprotective effects (11).
Nowadays, vibrational spectroscopy is recognized as one of the main tools that have made significant progress in the field of clinical diagnostics. This technique is simple and needs a small amount of the biological material and a reagent-free screening method. Fourier Transform Infrared (FTIR) is a type of vibrational spectroscopy that identifies structural moieties of biomolecules based on their IR absorption caused by the transition between the energy levels of molecular vibrational normal modes (12). It can be used to analyze macromolecules such as carbohydrates, amino acids, fatty acids, lipids, proteins, and nucleic acids. FTIR spectroscopy is increasingly used for diagnostic purposes by analyzing biofluids, tissue sections, or cells in various diseases (13-15).
2. Objectives
Thus, the present study aimed to evaluate the effect of irradiated and non-irradiated chicory root extract on liver injury induced by CCl4.
3. Methods
3.1. Chemical and Reagents
All chemicals used in this study, including CCl4, were purchased from Sigma-Aldrich.
3.2. Experimental Design
Twenty-four male Wistar rats of 180-200 g weight were purchased from the Razi Research Institute of Kerman, Iran, and kept at the Central Animal House, Department of Biology, University of Jiroft. Rats were housed in groups of four per cage and maintained under standard laboratory conditions (12 h light: 12 h dark and 24 ± 2ºC) during the experimental period. Free access to food and water was allowed at all times. All experimental protocols were done following the guidelines for the care and use of laboratory animals. The ethics code for the research project is IR.PNU.REC.1397.020. Animals were equally and randomly divided into six groups, as follows:
Group I): Animals in this group served as normal controls and were given water and food ad libitum during the study.
Group II): Rats were given IP injections of CCl4 (1 ml/kg b.w) dissolved in olive oil (2:3 v/v) twice a week for four weeks (16).
Group III): Irradiated chicory root extract (500 mg/kg b.w by gavage) was administered to CCl4-treated rats three times a week for four weeks (17).
Group IV): Non-irradiated chicory root extract (500 mg/kg b.w by gavage) was administered to the CCl4-treated rats three times a week for four weeks.
Group V): Irradiated chicory root extract was administered to normal rats with a dose of 500 mg/kg b.w three times a week for four weeks.
Group VI): Animals in this group received only non-irradiated chicory root extract with a dose of 500 mg/kg b.w three times a week for four weeks.
Treatments were done for four weeks. At the end of the study, all animals were sacrificed, and blood samples and liver tissues were collected for biochemical, histopathological, and FTIR examinations.
3.3. Plant Collection and Radiation Facility
Roots of C. intybus L. were collected from Jiroft. A voucher specimen of the plant was deposited in the Herbarium of Faculty of Biology, University of Jiroft (Reg no. 313), Jiroft, Iran. The roots were rinsed with tap water and then subjected to 8 kGy gamma radiation using a 60Co source (Gamma cell 220) at room temperature in the Atomic Energy Organization of Iran.
3.4. Plant Extract Preparation
After the radiation process, the irradiated and non-irradiated chicory roots were dried in the shade. Roots were extracted with 95% ethanol for 72 h and filterd by Watman No. 1. The extract was dried in a vacuum using a rotary evaporator, and finally, the dried extract was weighed. The plant extract was dissolved in distilled water to make the final concentration of 500 mg/ml before oral administration..
3.5. Serum Preparation
At the end of the study, blood samples were collected from all treatment groups. Blood was stored at room temperature for 45 min and then centrifuged at 3000 × g for 5 min. The serum was collected and kept at -20ºC for biochemical measurements.
3.6. Biochemical and Antioxidant Assay
Lipid peroxidation in serum was measured by Thiobarbituric Acid (TBA). Malondialdehyde (MDA) is the end product of lipid peroxidation, which forms a pink color complex with TBA with maximum absorption at 532 nm according to the method by Khadir et al. (18). Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT) activities in serum were determined, according to Ghadi et al. (19). Catalase activity was determined by the method of Weydert and Cullen (20). The activity of Superoxide Dismutase (SOD) was determined at 560 nm according to the method of Thomas et al. (21).
3.7. Histopathological Study
Liver segments of all treatment groups were fixed in 10% formalin and embedded in paraffin. Sections of 5 μm were stained with hematoxylin and eosin for histopathological observation at the light microscopic level (22).
3.8. FTIR Study
For the FTIR study, the livers of rats treated in different groups were obtained and washed with ice-chilled saline. The samples were first oven-dried at 70 °C to a constant weight and then ground in an agate pestle and mortar. Powdered samples were weighed and mixed thoroughly with potassium bromide (KBr) at the 1:10 ratio (5 mg of dry tissue powder + 50 mg of KBr). The mixture of tissue and KBr was ground until the sample became well dispersed in KBr and homogeneously distributed. The final mixture of the sample was then molded into self-supporting pellets. The mixture was pressed at a pressure of 10-15 tones with the help of a hydraulic pressure machine to obtain pellets. The pellets were transferred in a spectrophotometer sample holder. The FTIR spectra were recorded in the range of 400-3500 cm-1.
3.9. Statistical Analysis
All data are presented as mean ± Standard Derivation (SD). The statistical significance was determined using one-way Analysis of Variance (ANOVA), followed by a multiple post hoc test (LSD). Differences were considered statistically significant at P value of < 0.05, P value < 0.01, and P value < 0.001.
4. Results
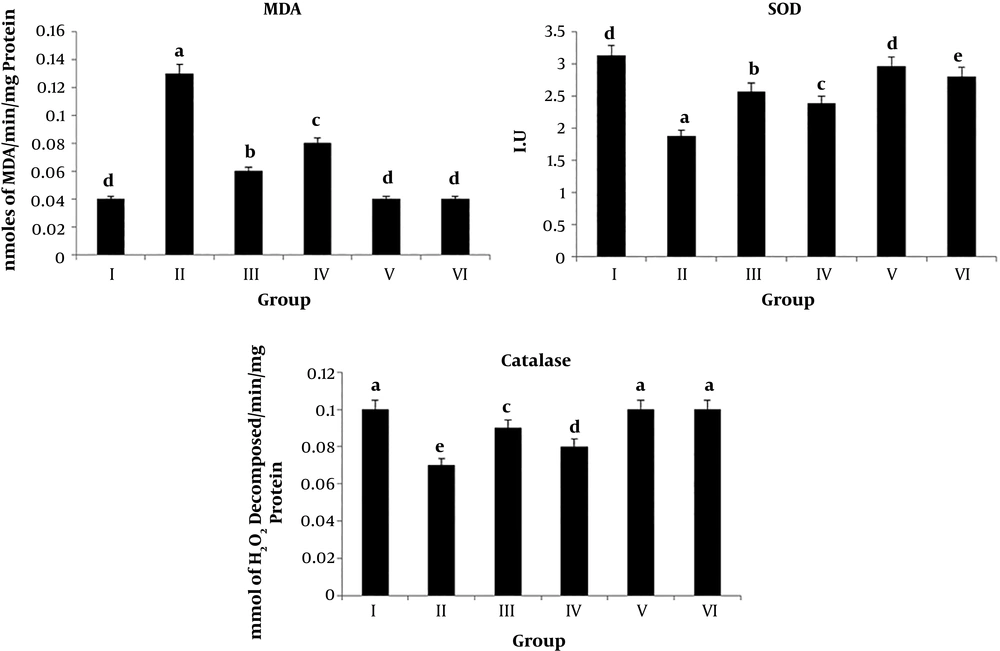
4.1. Lipid Peroxidation and Antioxidant Activities
Variations in lipid peroxidation and antioxidant activity of animals subjected to different treatments are shown in Table 1 and Figure 1. The MDA level is a direct indicator of lipid peroxidation, which showed significant increases (P value < 0.001) in CCl4-treated animals for four weeks. Moreover, irradiated and non-irradiated chicory root extract administration to normal rats did not induce any significant changes in the MDA level. Irradiated and non-irradiated chicory root extract administration to CCl4-treated rats significantly (P value < 0.01 and P value < 0.05, respectively) decreased the elevated levels of MDA. Figure 1 shows that the irradiated chicory root was more effective than the non-irradiated chicory root on the MDA level in CCl4-treated rats. Besides, CCl4 treatment on normal rats resulted in a significant decrease (P value < 0.001) in antioxidant enzymatic activities of catalase and SOD. However, irradiated chicory root extract supplementation to CCl4-treated rats resulted in a significant elevation in the activities of SOD (P value < 0.01) and catalase (P value < 0.05) when compared to the CCl4-treated group. Whereas, the non-irradiated chicory root extract was increased (P value < 0.05) the SOD level, but did not induce significant changes in catalase activity (Table 1 and Figure 1).
| Groups | Lipid Peroxidation (nmoles of MDA/min/mg protein) | SOD (I.U) | Catalase (mmol of H2O2 Decomposed/min/mg protein) |
|---|---|---|---|
| I | 0.04 ± 0.00 | 3.13 ± 0.37 | 0.10 ± 0.011 |
| II | 0.13 ± 0.02*** | 1.87 ± 0.16*** | 0.07 ± 0.00*** |
| III | 0.06 ± 0.03### | 2.57 ± 0.32*,## | 0.09 ± 0.01# |
| IV | 0.08 ± 0.02## | 2.38 ± 0.34**,# | 0.08 ± 0.01** |
| V | 0.04 ± 0.02 | 2.96 ± 0.26 | 0.10 ± 0.00 |
| VI | 0.04 ± 0.01 | 2.80 ± 0.40 | 0.10 ± 0.00 |
aValues are expressed as mean ± SD.
bI) normal rats, II) CCl4-treated animals, III) irradiated chicory root extract + CCl4, IV) non-irradiated chicory root extract + CCl4, V) irradiated chicory root extract administration, and VI) non-irradiated chicory root extract administration.
c*P value < 0.05, **P < 0.01, and ***P value < 0.001 by one-way ANOVA, followed by LSD test when the groups are compared with the control group; #P value < 0.05, ##P value < 0.01, and ###P value < 0.001 by one-way ANOVA, followed by LSD test when Group III and IV animals are compared with Group II animals (N = 4).
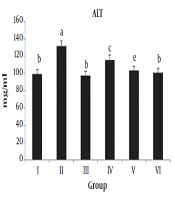
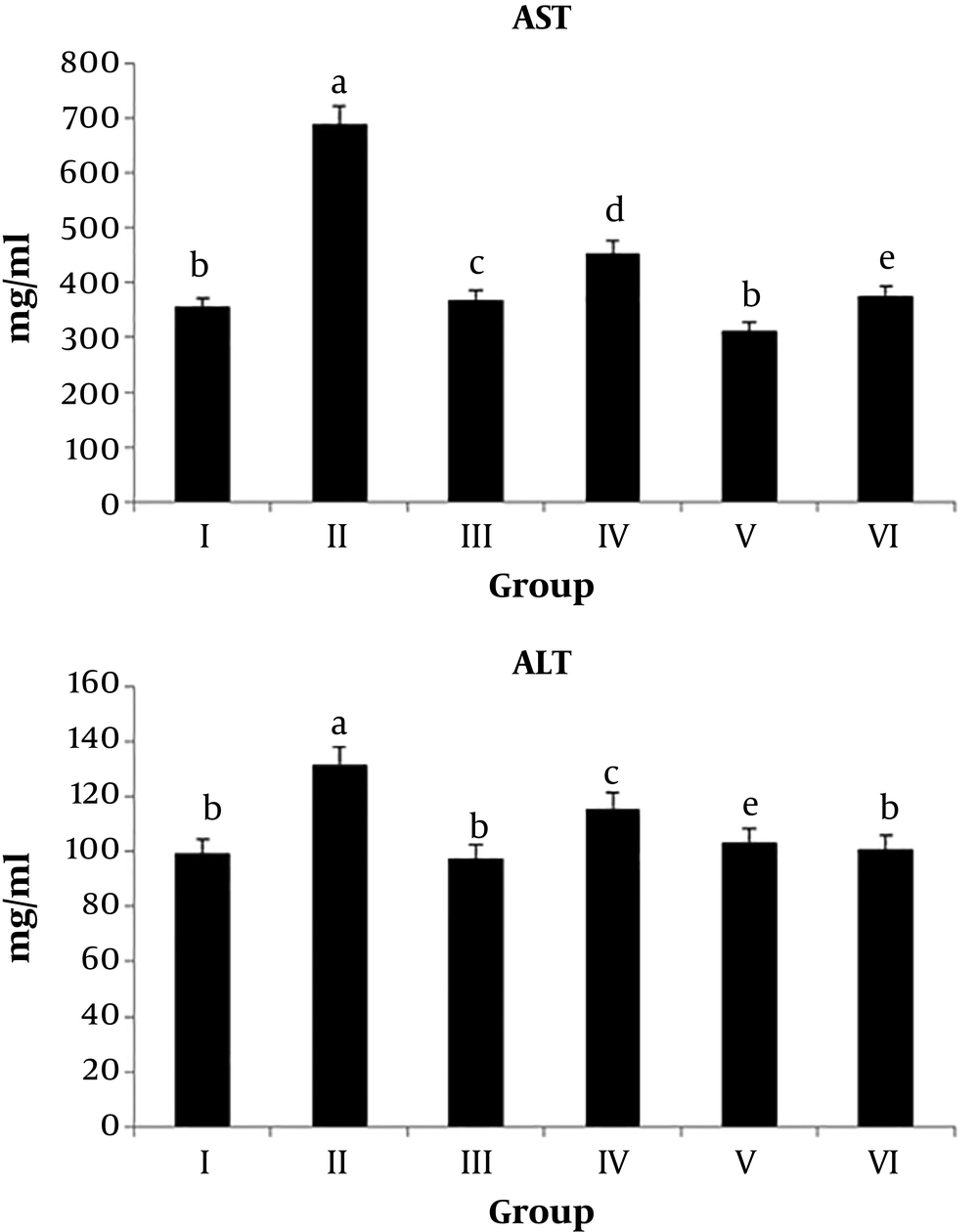
4.2. ALT and AST Levels
As shown in Table 2 and Figure 2, the serum ALT and AST levels were significantly higher (P value < 0.01 and P value < 0.001) in group II animals than in normal rats. The administration of the irradiated chicory root extract to CCl4-treated rats significantly decreased (P value < 0.01) the elevated levels of AST, but no significant changes occurred in the serum ALT level when compared to CCl4-treated rats.
| Groups | AST (mg/dl) | ALT (mg/dl) |
|---|---|---|
| I | 354.50 ± 69.39 | 99.50 ± 12.39 |
| II | 688 ± 185.63*** | 131.25 ± 9.94** |
| III | 367 ± 98.14### | 97.50 ± 18.52## |
| IV | 453.25 ± 98.17## | 115.50 ± 17.25 |
| V | 313.50 ± 87.17 | 103 ± 15.05 |
| VI | 374.50 ± 128.51 | 100.75 ± 16.72 |
aValues are expressed as mean ± SD.
bI) normal rats, II) CCl4-treated animals, III) irradiated chicory root extract + CCl4, IV) non-irradiated chicory root extract + CCl4, V) irradiated chicory root extract administration, and VI) non-irradiated chicory root extract administration.
c*P value < 0.05, **P value < 0.01, and ***P value < 0.001 by one-way ANOVA, followed by LSD test when the groups are compared with the control group; #P value < 0.05, ##P value < 0.01, and ###P value < 0.001 by one-way ANOVA, followed by LSD test when Group III and IV animals are compared with Group II animals (N = 4).
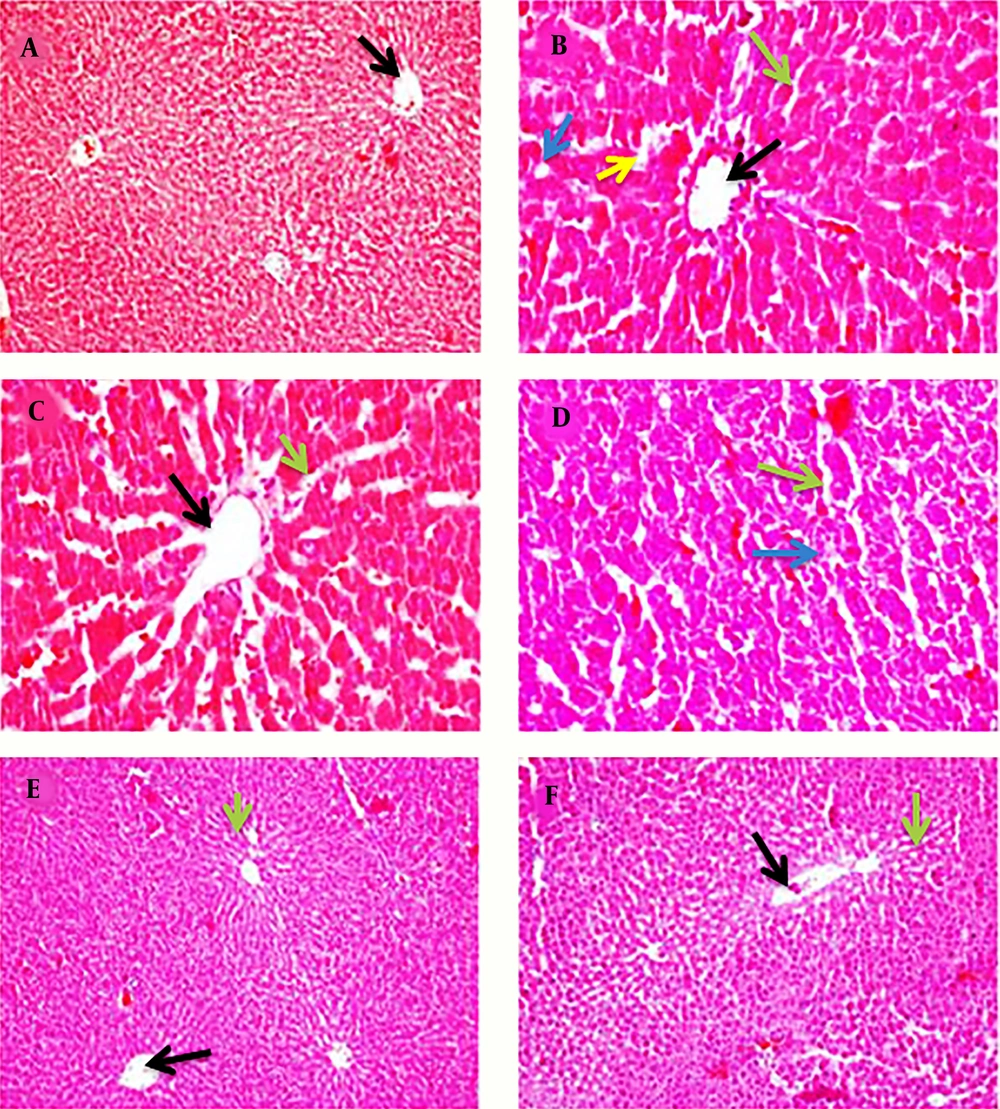
4.3. Histopathological Study
The results of the histopathological study are shown in Figure 3A to B. Liver histology in control, irradiated, and non-irradiated chicory root extract groups showed to be normal with hepatic lobular architectures consisting of hepatic cords of regularly arranged hepatocytes radiating from a central vein enclosing sinusoidal network (Figure 3A, E, and F). A liver section from the CCl4 group showed massive changes throughout the lobules, sinusoids with diffuse vacuolar cytoplasmic degeneration of hepatocytes, thickening of the epithelium, necrosis, and infiltration of mononuclear inflammatory cells (Figure 3B). Irradiated chicory root extract administration to CCl4-treated rats greatly restored the normal histoarchitecture in liver hepatocytes (Figure 3C). Combined treatments of non-irradiated chicory root extract with CCl4 showed mild to moderate lesions indicative of hepatocyte necrosis (Figure 3D).
Histo-Micrograph of Rat Liver From Different Treatment Groups. A: Normal histoarchitecture of rat liver (×10); B: Altered hepatocyte histoarchitecture of CCl4-treated rats (×40); C: Liver histoarchitecture of irradiated chicory root extract + CCl4-treated rats (×40); D: Histoarchitecture of non-irradiated chicory root extract + CCl4-treated rats (×40); E: Histoarchitecture of irradiated chicory root extract treated rats (×10); F: Histoarchitecture of non-irradiated chicory root extract treated rats (×10). Central vein (Black arrow), necrosis (blue arrow), sinusoid (green arrow), and ballooning degeneration of hepatocytes (yellow arrow).
4.4. FTIR Spectrum Analysis
The FTIR spectra in different treatment groups are shown in Figure 4. The FTIR analysis has proven to be a valuable tool for the characterization of functional groups. The normal control group showed 10 peaks in the range of 3297.59 to 698.11 cm-1 (Figure 4A). Moreover, the FTIR spectrum of non-irradiated chicory root extract also recorded the same number of peaks lying between 3293.72 and 697.18 cm-1 (Figure 4F). The FTIR spectrum of CCl4-treated animals showed broad changes in peak numbers (Figure 4B).
FTIR Study of Rat Liver In Different Treated Groups. A: Normal FTIR spectra of rat liver; B: FTIR spectra of CCl4-treated rats; C: Liver FTIR spectra of irradiated chicory root extract + CCl4-treated rats; D: FTIR spectra of non-irradiated chicory root extract + CCl4-treated rats; E: FTIR spectra of irradiated chicory root extract-treated rats; F: FTIR spectra of non-irradiated chicory root extract treated rats.
The FTIR spectrum of rats liver from group III with combined treatments of CCl4 and irradiated
chicory root and also group V which received irradiated chicory root extract during the experimental studies were revealed 11 peaks (Figure 4C and E); whereas, non-irradiated chicory roots supplementation to CCl4-treated rats recorded 10 peaks (Figure 4D).
The band in the 3297 cm−1 region arises from N–H and O–H stretching modes of proteins,
intermolecular hydrogen bonding, and also assigned to an amide I modes of proteins, which is shifting in CCl4 -treated rats (3289.26 cm−1). Irradiated and non-irradiated chicory root extracts were improved the changes to normal levels (3297.39 and 3292.28 cm−1, respectively). The prominent absorption at 1665 cm−1 arises from C-O stretching (amide I) and N–H bending (amide II) vibrations. The results showed that the bands at 1665.52 cm-1 were decreased (1649.81 cm−1) in CCl4-treated rats whereas, irradiated and non-irradiated chicory root extract treatments to the CCl4 group ameliorated most of the changes. The band located at 1041 cm−1 indicates the symmetric and anti-symmetric PO2- stretching vibrations of the DNA phosphodiester, which is shifted to 1080.41 cm−1 in CCl4-treated rats, when compared to the control group. However, the irradiated and non-irradiated chicory root extracts brought back the shifted peak to the control level. In the present study, the band area of 2931 cm-1 indicates the CH2 asymmetric stretching vibration and an acyl chain of fatty acids, which is increased to 2960.78 cm-1 in CCl4-treated rats.
Irradiated and non-irradiated chicory root extracts were proved the altered band (2960.78 cm-1) in the CCl4 group. The band observed at 1452 cm–1 is due to the bending vibration of CH2 in lipids and proteins. Figure 4B shows that the band was removed in the CCl4 group. The band area of 698cm-1 presents the C-O and C-C group vibration modes of carbohydrates, which is absent in CCl4-treated rats.
The irradiated and non-irradiated root extracts treatments to the CCl4 group were ameliorated the changes at the band area of 1452 and 698 cm-1.
5. Discussion
The liver is a sensitive vital organ that plays a major role in clearing the chemicals and is a target of toxicity (23). Hepatotoxicity is the most widespread pathology worldwide and the most increasing cause of health problems (24). The medicinal plants have recently been used in protecting against or treatment of liver damage. Chicory is one of the traditional medicines used for different problems. Secondary metabolites are responsible for the antioxidant activity of chicory root, as reported in different studies (25).
The results of previous studies from our lab showed that the phenolic contents of irradiated chicory roots increased under the effect of low-dose gamma irradiation (8 kGy) (26). These results are in agreement with other research that reported that gamma radiation dose up to 10 kGy increased phenolic contents, antibacterial, and free radical scavenging activities of plants (27, 28). The present study demonstrated that CCl4 is a liver toxicant that changes serum biochemical parameters related to the liver and antioxidant defense function. The results obtained from our study showed that the serum levels of ALT, AST, and MDA were significantly higher, and the activities of SOD and catalase antioxidants were lower in group II than in the control group. A significant increase was reported in the serum ALT and AST levels in CCl4-treated rats (29). The study demonstrated that CCl4 is associated with increased lipid peroxidation and decreased SOD and catalase activities (30). The increase in lipid peroxidation and the decrease in antioxidant activities are indicative of CCl4-induced oxidative stress. However, the irradiated chicory root extract decreased the elevated level of ALT and AST in the CCl4 group. The study showed that the administration of chicory root extract to CCl4-treated rats induced hepatoprotective effects. Liver histology of CCl4-treated rats showed marked structural alterations due to induced oxidative stress. Another study also revealed liver structural changes in CCl4-treated rats (31).
Also, the FTIR spectra showed broad changes in the functional group of DNA, proteins, lipids, and carbohydrates in group II. In the present study, the normalization of molecular and structural changes was observed in irradiated and non-irradiated chicory root extract administrated CCl4 groups. Cichorium intybus is a good source of secondary metabolites due to higher total phenolic and chlorogenic acid contents and has been found to possess good DPPH radical scavenging capacity and antibacterial activity, which may be responsible for its observed antioxidant activities. Gamma irradiation increases the production of phenolic contents as observed in Pterocarpus santalinus when subjected to different doses of gamma radiation (32).
In conclusion, the present study demonstrated that irradiated and non-irradiated chicory root extracts possess potent antioxidant activities. Moreover, the results showed that the irradiated chicory root extract was more effective than non-irradiated chicory root extract. This could be explained by the effect of gamma-ray on the phenolic content. The low-dose gamma-ray can increase the free radical scavenging and antioxidant activities of the chicory root.