1. Background
Over the years, a wide variety of fermented foods have been prepared from the milk of animals such as camels, cows, buffaloes, and goats by people around the world. Since about 8000 years ago that animal husbandry started by humans, the production of this type of food has changed, and the historical way of preparing fermented milk products has been created (1, 2). Traditional dairy product “Shoor” is one of the most significant components of the family of traditional dairy products in the region of Azerbaijan. This dairy product is made from sheep’s milk. It has a texture similar to grated cheese. Its special smell and taste well indicate the traditional nature of this dairy product.
Owing to the probiotic activity of fermented milk products existing organisms, these products impart nutritional and health benefits to their consumers (1-3). There have been many studies around the world, showing that some dairy products and natural foods may have anticancer effects (4).
The most important microorganisms involved in food fermentation are lactic acid bacteria (LAB) (5). The use of LAB in many dairy products represents the fact that they are non-pathogenic and safe for humans as oral administration. This causes the LAB to be considered living carriers of oral or local vaccines (6). Over at least 4,000 years, LAB has been used to ferment foods such as cheese, yogurt, etc (7). Among LAB, the most widely used microorganisms are the Lactobacillus genus because these are beneficial microorganisms. People around the world have extensively used lactobacilli to produce food and protect against germs that could cause food poisoning or spoilage. Today, due to the beneficial effects (functional properties) of these bacteria, the use of Lactobacillus is essential in the food industry (1, 8). The ability of lactobacilli to protect fermented foods from spoilage is mainly related to producing acid during their metabolism in fermented foods. Converting carbohydrates to organic acids is effective in improving the quality and increasing the durability of these foods by increasing pH (9). Lactobacilli are used in the industry to modify the flavor, taste, and texture of fermented products, and these bacteria or their pure bacteriocins are used as biological preservatives in foods due to the effect of inhibiting the growth of various bacteria (8, 9). In addition to the above properties, recent research suggests that lactobacilli may play a significant role in reducing the risk of cancer incidence. The results of laboratory studies also indicate the anticancer effects of these bacteria (10).
2. Objectives
This study aimed to evaluate anticancer activities of lactobacilli in traditional dairy products.
3. Methods
3.1. Isolation of Lactobacilli
To isolate the lactobacilli, samples were taken aseptically from the traditional dairy product “Shoor” and were transferred to the laboratory. The samples were diluted by sterile physiological serum. For this purpose, 1 g of each sample was completely dissolved separately in 9 mL of sterile physiological serum. After preparing one-tenth dilution in this way, one-hundredth and one-thousandth dilutions were also prepared. Then, 0.1 mL from obtained suspensions was cultured on MRS (De Man, Rogosa, and Sharpe) medium (HiMedia-India). The culture plates were inoculated for 48 hours at 37ºC. After this time and the emergence of colonies on the plates, for the initial studies, the Gram staining and catalase test were performed, and Gram-positive bacilli were cultured purely (11).
3.2. Supernatant Preparation
To this purpose, the MRS broth medium (BRlife-Italy) was used. Subsequently, 100 µL of fresh bacterial cultures were inoculated on this medium, and the cultures were transferred to a 37ºC incubator for a 24-hour incubation. Then, cultures’ OD (Optical Density) at 600 nm was adjusted to 1.25. To obtain the supernatant containing the metabolites, the cultures were centrifugated at 3,000 rpm (revolutions per minute) for 30 min. By discarding the bacterial pellet, the supernatant was collected and was sterilized by a 0.22-micron filter for cell toxicity analysis (8).
3.3. Identification of Lactobacilli
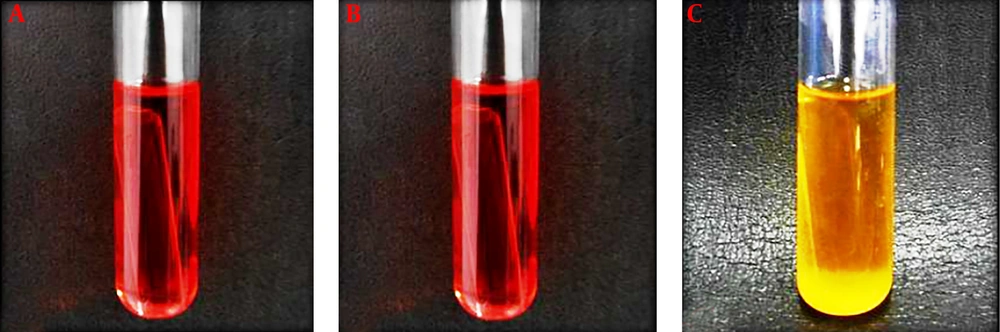
Biochemical identification: After Gram staining as well as catalase testing to isolate Gram-positive and catalase-negative bacilli, biochemical tests were performed to diagnose Lactobacillus species. During this phase of detection, Vogues-Proskauer (VP), carbohydrate fermentation of glucose, galactose, lactose, arabinose, maltose, mannitol, sorbitol, sucrose, xylose, melibiose, raffinose, and trehalose sugars (in phenol red broth medium with 1% sugar), the dehydrolysing of arginine reaction and the monitoring of growth at 15ºC and 45ºC were performed (5, 12, 13).
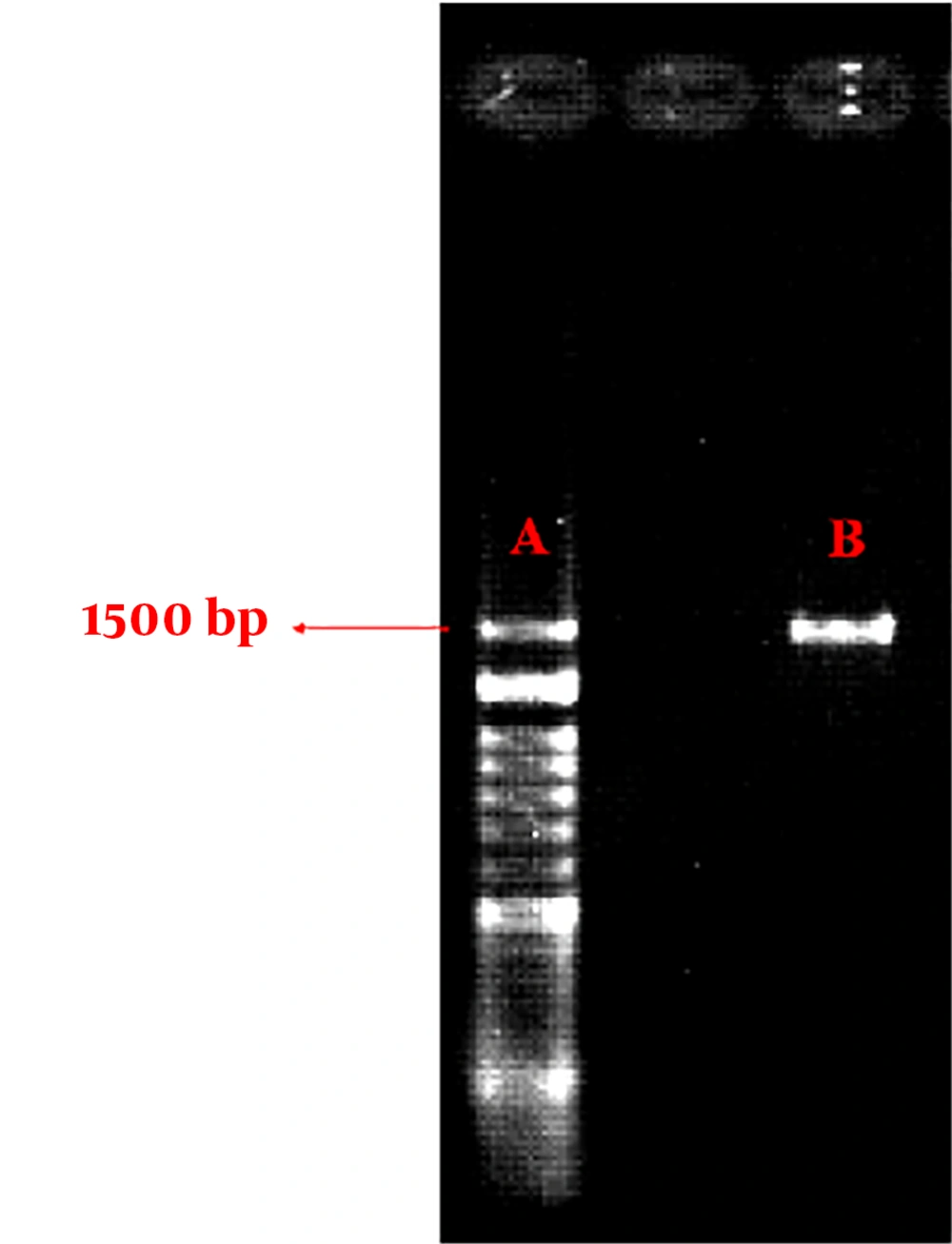
Molecular identification: Polymerase Chain Reaction (PCR) was performed to more accurately identify the desired strain. To this purpose, the genomic DNA of the isolates was extracted, and the PCR reaction using the universal primer and agarose gel electrophoresis was performed with the aim of multiplying and sequencing the 16S rRNA gene. The sequencing was performed by Bioneer-Germany and analyzed by the Basic Local Alignment Search Tool (BLAST) of the National Center for Biotechnology Information (NCBI) site (http//www.ncbi.nim.nih.gov/BLAST) (14-16), and the dendrogram of the isolated bacteria was created by Mega 5 software.
3.4. Cell Analysis
Cancer cell culture: HCT116 colorectal cancer cells were purchased from the Pasteur Institute of Iran cell bank and were cultured in Roswell Park Memorial Institute 1640 (RPMI1640) (Gibco, England) medium with 10% Fetal Bovine Serum (FBS) (SIGMA-Germany) and 1% Penicillin and streptomycin mixture (SIGMA-Germany) at 37ºC and 5% CO2 (17).
Cell survival: In order to test the cell toxicity of the obtained metabolite, MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) assay was performed, and the viability of the cells treated with the metabolites was determined. For this purpose, the amount of 104 HCT116 cells per well were cultured in a 96 wells plate, containing 200 μL of complete cell culture medium and were treated with 0.5, 1, 1.5, 2, and 5 × 107 CFU (colony forming unit) per ml concentrations of bacterial metabolites, respectively for 24, 48 and 72 hours at 37ºC and 5% CO2. The first well of each row was designated as negative control (no treatment). After this time, 20 μL of MTT solution (5 mg/mL) was added to each well. After a 3-hour incubation, the top liquid of the plates was drained, and 200 μL of Dimethyl Sulfoxide (DMSO) per each well was used for solubilization of the blue formazan crystals. The absorbance of the solution was measured using an ELISA (enzyme-linked immunosorbent assay) reader at 570 nm. The results were analyzed, and the cell survival rate was calculated at different concentrations and time intervals (17-19). The results of the MTT assay were analyzed with the statistical package for social science (SPSS) software using “ANOVA and Tukey” tests.
4. Results
4.1. Isolation of Lactobacilli Results
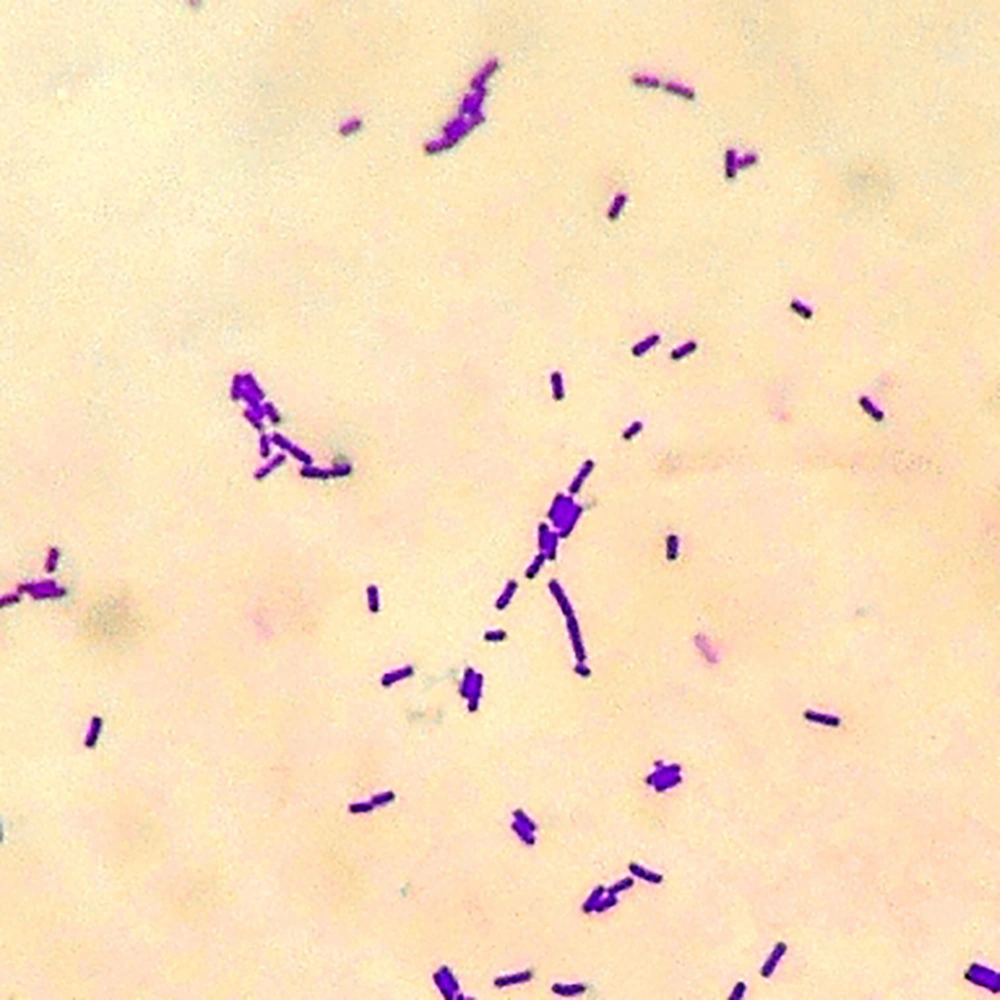
Three isolates were isolated from the traditional dairy product and were coded as ST1, ST2, and ST3. The isolates were Gram-positive (Figure 1) and catalase-negative.
The results of biochemical tests related to ST1 isolate are shown in Tables 1 and 2 and Figure 2. The results of the PCR test are shown in Figure 3.
| Characteristics | Value |
|---|---|
| Gram | + |
| Catalase | - |
| V-P | - |
| Arginine Dehydrolysis | + |
| Growth in 15 and 45ºC | +, + |
| Variable | Value |
|---|---|
| Glucose | + |
| Galactose | + |
| Lactose | - |
| Arabinose | + |
| Maltose | + |
| Mannitol | + |
| Sorbitol | + |
| Sucrose | - |
| Xylose | + |
| Melibiose | + |
| Raffinose | - |
| Trehalose | + |
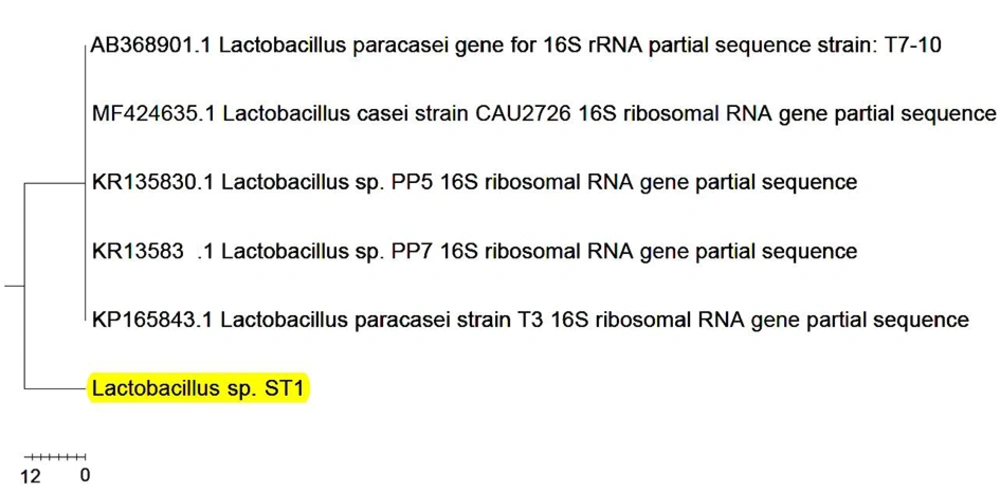
Comparison of Lactobacillus sp. ST1 sequences on the NCBI site showed 99% genetically relating to the isolate to Lactobacillus paracasei (Figure 4). The sequence of this strain was recorded in NCBI with MF506843 code.
4.2. Cell Analysis Results
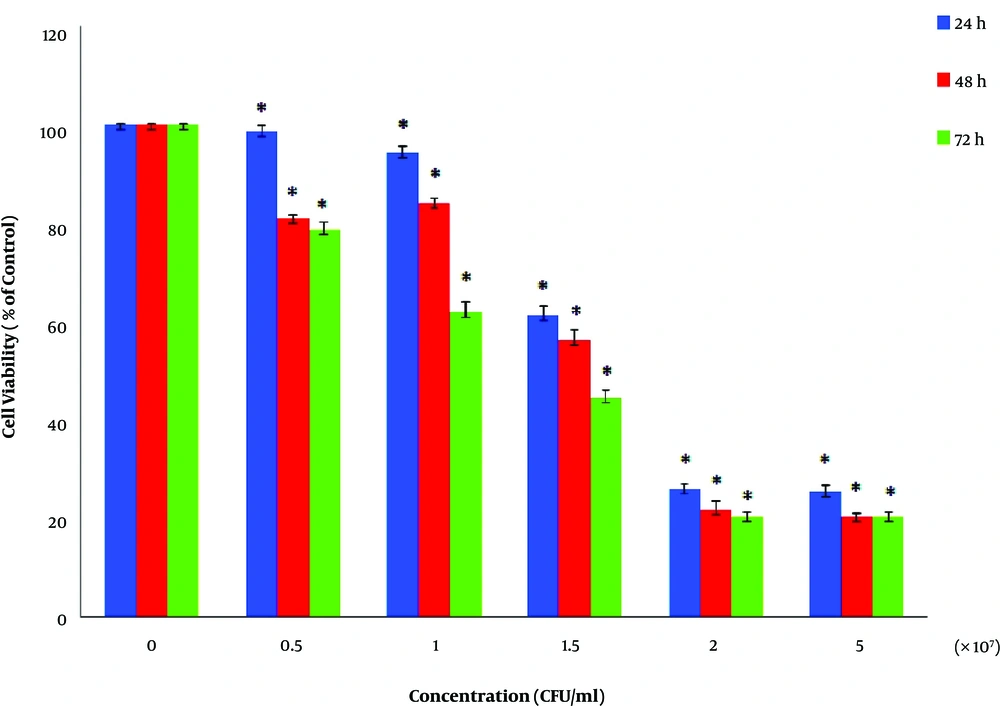
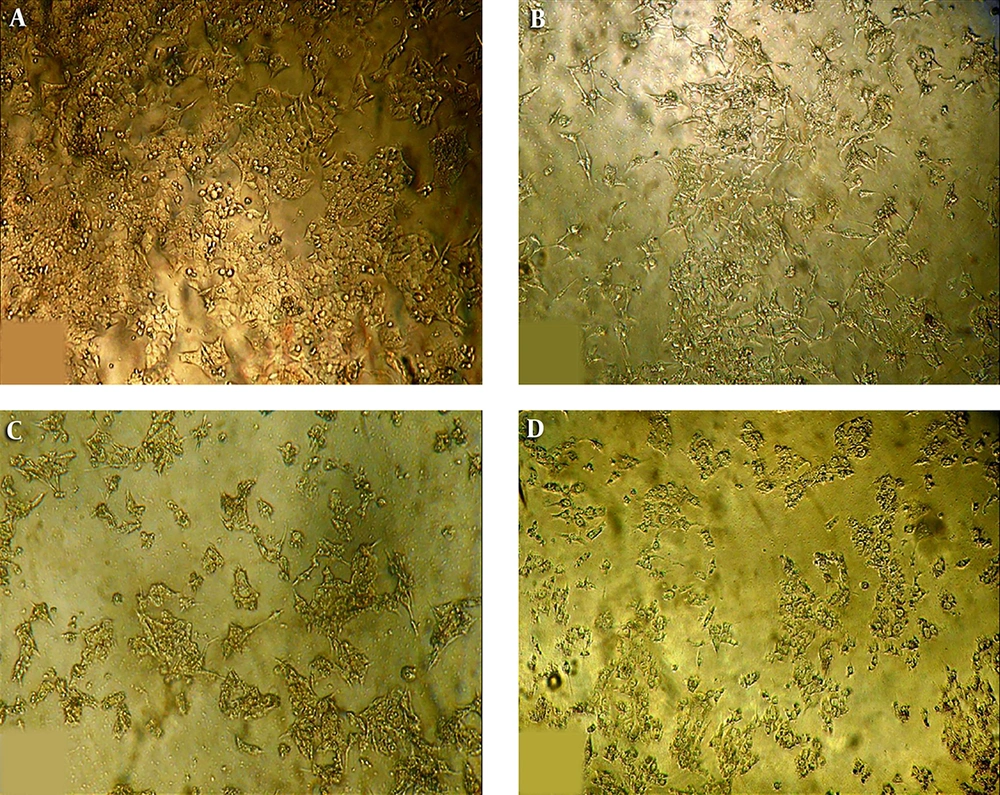
The cell survival and MTT assay results of HCT116 cells are shown in Figure 5. Accordingly, the ST1 strain supernatant has an anti-proliferative and cytotoxic effect on the cancer cell line. In this regard, the IC50 value of 72 hours related to HCT16 cells was calculated to be equal to 1.35 × 107 CFU/mL. All values and results are presented based on the mean ± SD of three independent repetitions, and P < 0.05 was considered the level of statistical significance. The images obtained from the inverted microscope (Figure 6) showed that the treated cells with an IC50 value of supernatant appear wrinkled, and after continuing (increasing time), the rupture and fragmentation of cells were observed.
5. Discussion
In this study, the cytotoxic anti-proliferative effects of Lactobacillus paracasei ST1 isolated from the “Shoor” traditional dairy product were evaluated. The cytotoxic effect of the metabolite obtained from the strain was observed on the studied cell line. Studying of the MTT assay results showed that the cytotoxic effect of the studied metabolite was dose- and time-dependent, and with increasing these parameters, the decrease in cell survival was observed. A comparison of the IC50 value of 72 hours related to HCT16 cells with results of other studies shows that the studied metabolite can offer significant cytotoxic effects. Similar studies have been conducted in this field. Choi et al. performed a study in 2005 that indicated that the extract of Lactobacillus acidophilus (108 CFU/mL) inhibited the treated cells 21% - 28% compared to untreated cells (20). A comparison of these results with the obtained value in our study shows that the obtained extract in Choi et al. study has a much lower inhibitory effect. In 2011, Kabiri et al. showed that the cytoplasmic extracts of Lactobacillus paracasei and Lactobacillus casei were able to appreciably inhibit the growth of K562 cancer cells (21). Owing to the different methods of extraction and dilution, it is impossible to accurately compare the results of this study with the present study. In 2014, Sadeghi-Aliabadi et al. showed that Lactobacillus plantarum A7 supernatant has a significant inhibitory effect on Caco-2 colorectal cancer cells (17). In 2015, Tuo et al. investigated the effect of eight different strains of Lactobacillus on K562 cancer cells, with different results that the anticancer effect was observed in all strains (22). The examination of the inhibitory effects of the Lactobacillus acidophilus supernatant with a concentration similar to our project on the Caco-2 cells performed by Soltan Dallal et al. in 2015 showed that the cytotoxic effects were no more than 38% (18) while in the present study, the cell survival rate in 5 × 107 CFU/mL concentration was below 20%. This also confirms the significant effects of studied metabolites in the present study. In the Kahouli et al. study in 2015 that evaluated the anti-proliferative effect of Lactobacillus fermentum supernatant on colorectal cancer cells (19), they indicated that the results were almost consistent with the results of the current project. In 2015, Er et al. examination results on the cytotoxic effects of many Lactobacillus species on the Caco-2 cells were not considerable (23).
Following up and using the correct methods for metabolite obtaining, which make release metabolites in a suitable and quality amount, may be the reasons for the high cytotoxic effect of the obtained compounds. In the meantime, it is necessary to pay much attention to the possibility of high anticancer activity of the probiotics of traditional dairy products compared to other probiotics.
5.1. Conclusions
The human diet has never been devoid of valuable nutrients such as dairy products. In many countries around the world, the use of these products has always been strongly recommended. (3). Considering the results of this project, focusing on the beneficial effects of lactobacilli in traditional dairy products will be a promising approach to discovering new and effective anticancer compounds. Traditional dairy products in the Azarbaijan region contain a significant amount of quantitative and qualitative diversity of beneficial lactic bacteria, and if people’s awareness of the high use of these products in society increases, the tendency to use this type of natural food will increase. As observed, the “Shoor” traditional dairy product has the potential to isolate organisms with significant probiotic properties, and the search for the use of these microorganisms as a starter is one of the important points that should be considered in future studies.