1. Background
It is more than sixty thousand years that medicinal plants are used by humans. These plants are great sources for obtaining medicines. Moringa oleifera (MO) is a fast-growing tree that is widely cultivated in northwestern India (Southern Himalayas) and now can be found throughout the tropical belt. This fast growth tree is more in Sistan and Baluchistan, and Bushehr provinces of Iran (1).
Different parts of the MO plant have tremendous power in treating various diseases, especially liver diseases. For example, the effect of MO leaf on liver lipid-lowering gene expression has been investigated, and it’s proved that its compounds inhibit the production and accumulation of fat in the liver (2). Also, the ethanolic extract of this plant can reduce acidification rate, gastric ulcer, and stomach pH. The results of this study showed that the ethanolic extract of MO root has an anti-inflammatory effect (3). Another study has compared the antioxidant potential of leaf and seed of MO and reported that flavonoid content and antioxidant properties of MO leaf powder were significantly higher than its seed powder (1, 4, 5). Among the variety of effective pharmaceutical substances, flavonoids are of particular importance and constitute a large group of secondary metabolites. These substances are present in almost all plants. The number and position of the hydroxyl phenolic group affect the antioxidant activity of these compounds (6, 7).
The antioxidant activity mechanism of flavonoids can be defined by direct scavenging or quenching of oxygen free radicals or excited oxygen species. Moreover, flavonoids inhibition of oxidative enzymes generates these reactive oxygen species (ROS) (8).
Quercetin is an important flavonoid that can be found in plant compounds. Also, quercetin inhibits oxidative damage through various mechanisms such as scavenging of oxygen free radicals and preventing lipid peroxidation (3, 9). Quercetin stabilized oxygen by releasing one electron to reactive oxygen species (ROS). Quercetin has an important role in the production of glutathione as an antioxidant that causes a (ROS) neutralizing agent. Quercetin increases the activity of the glutamyl cysteine synthase (GCS) enzyme, which is a key enzyme in glutathione synthesis. Also, quercetin increases expression (Nuclear factor-like 2) of the transcription factor. Nrf2 is a transcription factor involved in the defense system against oxidative stress-related damage and, when activated, increases the production of proteins involved in the process of reducing oxidative stress in the cell (10).
Liver cancer is one of the most common malignancies with high morbidity and mortality rates. Annually nearly one million people develop liver cancer worldwide and have a high mortality rate (11). Chemotherapy is a therapy that uses chemical drugs to kill cancer cells. It uses in cases that surgical interventions cannot be implemented, or the patient did not respond to other treatment methods such as radiotherapy. Given the disadvantages of chemotherapy, including the toxic side effects, the development of resistance to chemicals and etc., efforts to find drugs with natural origins that have less serious side effects are worthwhile (12). Several studies have indicated the anticancer activity of quercetin. It has been reported that quercetin at various concentrations causes tumor growth arrest of various cancer cell lines, including breast, colorectal, stomach, head and neck, lung, ovarian, melanoma, and leukemia.
2. Objectives
Given the medicinal properties and the effects of quercetin on the liver, for the first time, we identified quercetin as a flavonoid component of Moringa oleifera methanolic extract by HPLC and then focused on the evaluation of anticancer properties of MO methanolic extract on the HepG-2 cell line. In the following, these properties were compared with anticancer medicines such as doxorubicin and pure quercetin antioxidant.
3. Methods
3.1. Moringa oleifera Leaves Extraction) Preparation Herbal Extracts)
The Moringa oleifera leaves were purchased from Royan Dezful Green Company (Khuzestan, Iran) and were confirmed in the herbarium of the University of Isfahan by genus and species. About 25 g of leaves were dried for 24 h in the oven at 40°C and then were powered using electrical masonry. After performing the grinding process, the powder was passed through the 80-mesh screen. Extraction was performed using 250 mL of 99% methanol (Merck, Germany) (ratio 1: 10). The solvent extraction method was soxhlet. For this purpose, the sample was poured into Watman’s filter paper and placed into a soxhlet apparatus. In the following, the solvent was dumped into the balloon, and the heater temperature was in the range of medium to high setting. The excess solvent was separated by rotary evaporator (HANAVAPOR HS-2005S-N with vacuum pump N VE 180) at 50°C. The resulted extract was collected and kept away from the light at -20°C until further use (13).
3.2. Determination of Total Flavonoid Content by Spectrophotometric Assay
The modified method of Meda et al. (14) was used to determine the total flavonoid content. For this purpose, 0.5 mL of 2% aluminum chloride solution (Merck, Germany) in 99% methanol (Merck, Germany) was added to 0.5 mL of the extract. The mixture was stirred at room temperature for 5 minutes. The absorbance of the sample was measured using the spectrophotometer (SHIMADZU UV-1240) at 415 nm (14). To draw the standard curve, quercetin (Sigma-Aldrich, USA) was used. Then, using the quercetin calibration curve, the total flavonoid content of Moringa oleifera (MO) leaves extract (µg/g), and quercetin content of (MO) leaves extract (µg/g) were calculated using the equation of the line (y = 0.029x + 0.105) and reported as µg/g of extract (Table 1).
| Extract Concentration, µg/mL | |||||
|---|---|---|---|---|---|
| 70 | 60 | 50 | 40 | 30 | |
| Total flovonoid, µg/g | 0.2589 ± 0.0011A | 0.2250 ± 0.0025B | 0.1848 ± 0.0046C | 0.1479 ± 0.006D | 0.1152 ± 0.028E |
| Total quercetin, µg/g | 0.0045 ± 0.0012A | 0.0039 ± 0.0010B | 0.0032 ± 0.0011C | 0.0025 ± 0.0014D | 0.0019 ± 0.0012E |
avValues are expressed as mean ± SE.
bThe (a, b, c, d, e) letters indicate a statistically significant P < 0.05 between the different groups by Duncan test.
3.3. Identification and Calculation of Quercetin In Methanol Extract by High-Performance Liquid Chromatography (HPLC)
To prepare a standard solution for HPLC and standard curve drawing, first, 0.001 g of standard quercetin (Sigma-Aldrich, USA) was mixed with 5 mL of 99% methanol (Merck, Germany), and filtered with a 0.2-micron filter. Other standards were prepared from this standard solution and injected into the device with a syringe. Samples were analyzed by single-pump HPLC equipped with a single pump and detector (Agilent 1100, SY-8100 UV/VIS). The mobile phase consisting of 50% methanol and 50% acetonitrile at a specified flow rate (0.9 mL/min) was passed through a C18 column (4.6 × 250 mm). The absorbance of the samples was measured at 370 nm (13).
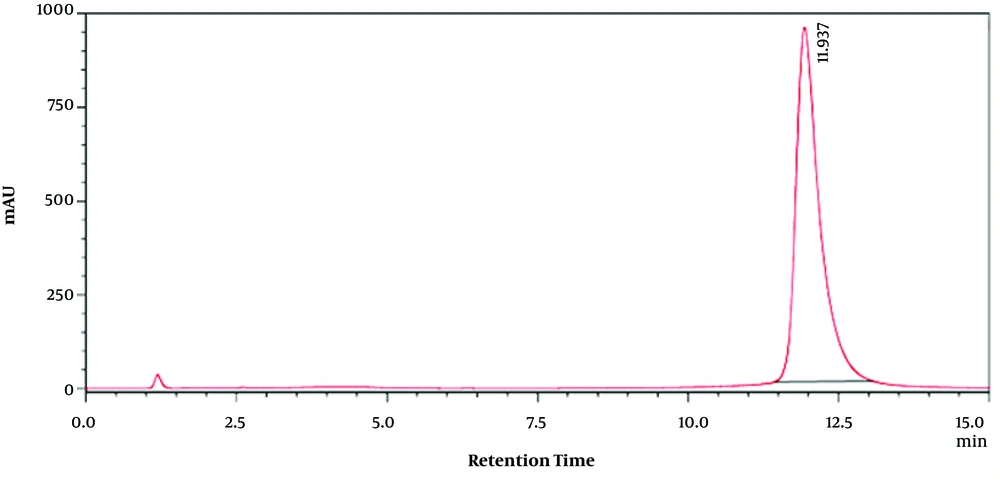
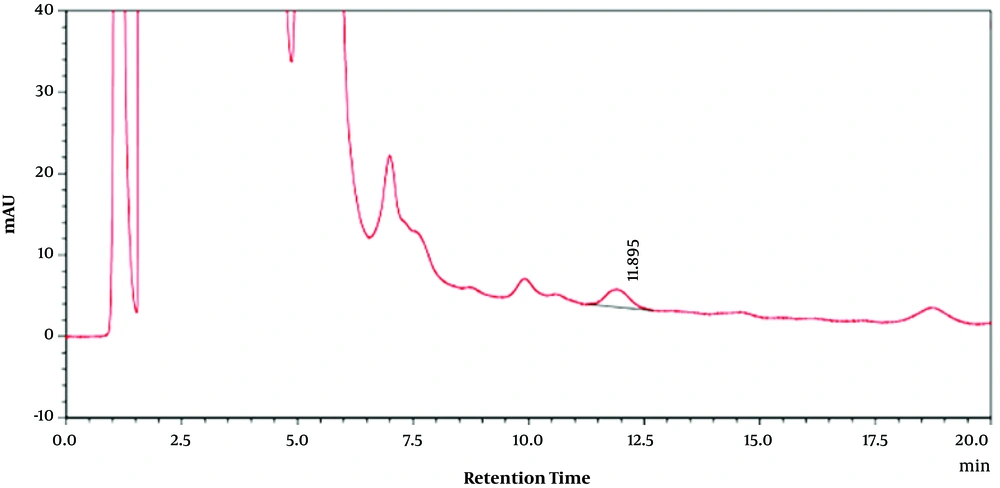
For extracts, 5 mL of Moringa olifera leaf methanolic extract solution was passed through a 0.2-micron filter and injected into the apparatus. Using obtained retention time on chromatograms of pure quercetin, the amounts of MO methanolic leaf extracts flavonoid compounds at different concentrations were calculated. Quercetin-related HPLC chromatogram as the used standard at 50 (µg/mL) is shown in Figure 1. Furthermore, Moringa olifera leaf methanolic extract HPLC chromatogram at 30 (µg/mL) is shown in Figure 2.
3.4. Cell Culture
HepG-2 (hepatocellular carcinoma) liver cancer cells were purchased from the Pasteur Institute Cell Bank (Tehran, Iran) and maintained in DMEM medium (Gibco, USA). All cell culture stages were performed in sterile conditions under the hood and in the cell culture room. Hep-G2 Cells were incubated in 25 cm2 cell culture flasks in 5 mL DMEM culture medium, 10% bovine fetal serum, 50 µg/mL streptomycin, 50 µg/mL penicillin, and 2 µg/mL amphitrecin in an incubator at 37°C and 5% CO2 were cultured.
The status of cells was observed under reversed-phase microscopy every day, and the cells were frozen in a logarithmic growth phase in a freezing medium and stored in liquid nitrogen at -196°C. The effects of various concentrations of MO leaf extract on Hep-G2 cell viability were investigated by plating Hep-G2 cells (104/well) in 96-well plates and incubating the cells in DMEM supplemented. After 24 h, the cells were washed once with medium and treated with 0, 30, 40, 50, 60, and 70 µg/mL MO leaf extract and doxorubicin (anticancer drug) (1 µg/mL) and pure quercetin (50 µg/mL). The control wells, including Hep-G2 cells, were cultured without the addition of MO extract, and blank wells containing only culture medium were established. Cell proliferation and viability were evaluated after 48h of treatment by incubating the cells in DMEM and 20 µL MTT (5 mg/mL) for 3 h. When the MTT dye was removed from the wells, 160 µL l of DMSO solvent was added to each well. The plate was wrapped in aluminum foil and stirred for 20 minutes. The optical absorption of the solution was obtained at 570 nm and read using a microplate reader (BIOHIT, BP 800), and cell viability was calculated using the following equation (Figures 3 and 4).
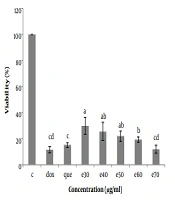
The cell viability (%) of Hep-G2 in culture with all concentrations of Moringa oleifera methanolic extract (e30, e40, e50, e60, and e70 µg/mL) and doxorubicin (1 µg/mL) and pure quercetin (50 µg/mL) in comparison with control significantly decreased. Data are reported as (mean ± SE) (n = 3). The dissimilar letters (a, b, c, d, e) on the columns indicate a statistically significant difference by Duncan test (P < 0.05). C, control; dox, doxorubicin; que, quercetin; e, extract.
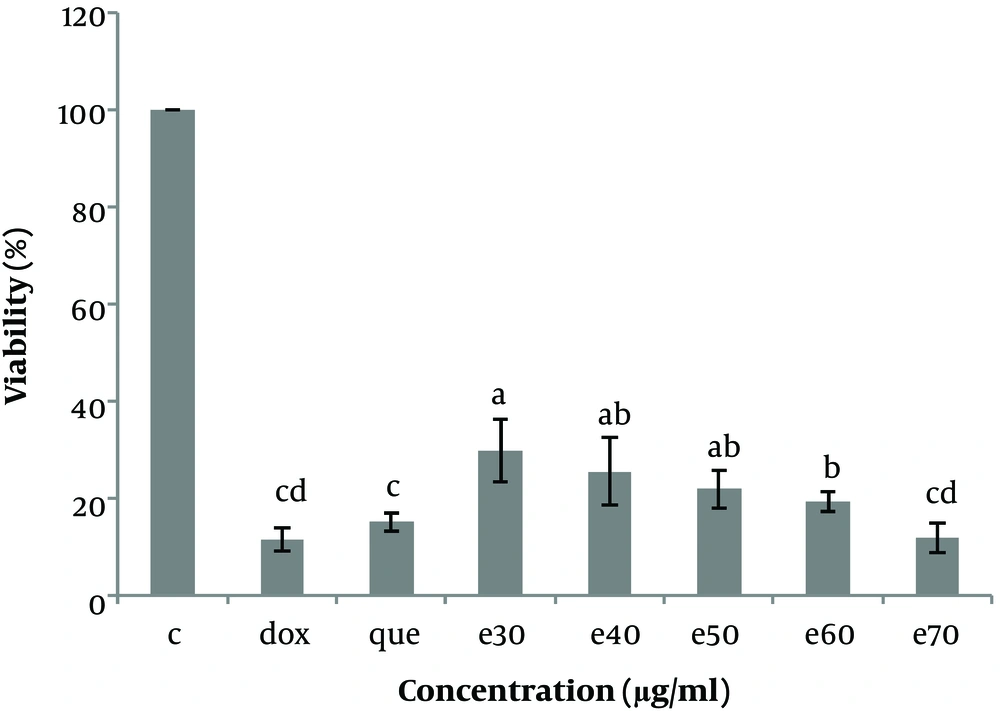
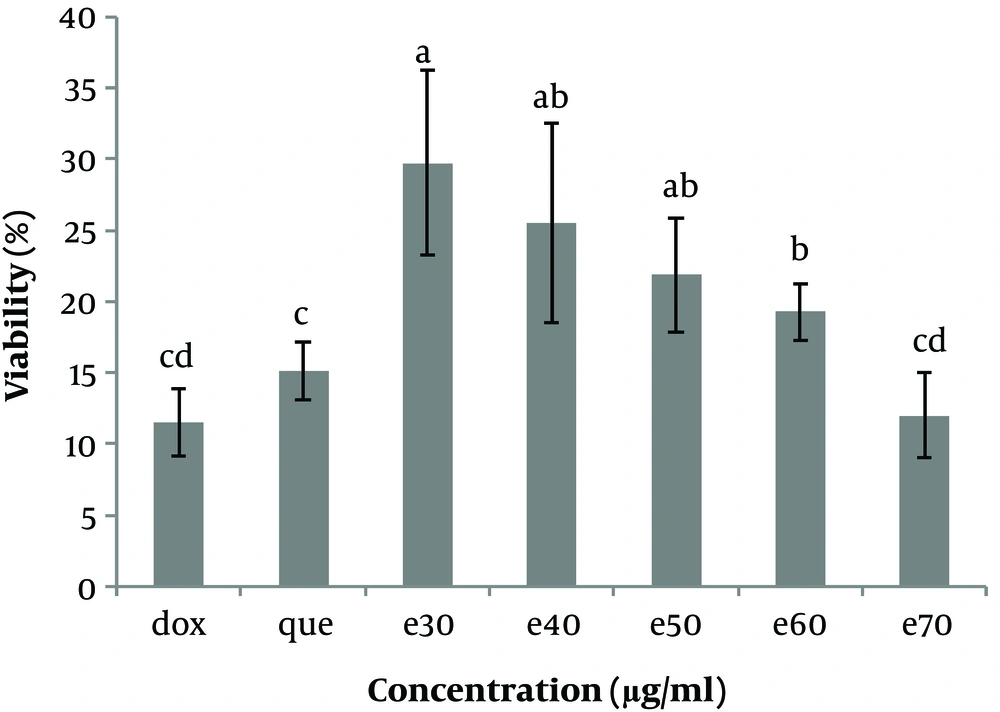
The cell viability (%) of Hep-G2 in culture with all concentrations of Moringa oleifera methanolic extract (e30, e40, e50, e60, and e70 µg/mL) in comparison with doxorubicin (1μg/ml) and pure quercetin (50 µg/mL). Data are reported as (mean ± SE) (n = 3). The dissimilar letters (a, b, c, d, e) on the columns indicate a statistically significant difference by Duncan test (P < 0.05). dox, doxorubicin; que, quercetin; e, extract.
100 × (mean light absorption in the control group/mean light absorption in the test group) = cell viability
3.5. Statistical Analysis
In this study, all experiments were performed with three replications. Experiments regarding the MTT were performed in a completely randomized design, and the results were expressed as the percentage of viability. 50% cell viability (IC50) was calculated using the Graph Pad Prism software. Analysis of variance was performed using SPSS software, and mean comparisons were made using Duncan’s multiple range test at the 5% level of probability.
4. Results
First, the content of phenolic compounds of Moringa olifera leaf extract was measured, and the standard curve and resultant equation were calculated for the concentration of flavonoid compounds at concentrations of 30, 40, 50, 60, and 70 µg/mL (Table 1). Flavonoid concentration in one gram of plant leaf was 3.69 (mg/g).
Then, the presence of quercetin as one of the important flavonoid compounds in methanolic extract of the plant leaf was confirmed by high-performance liquid chromatography (HPLC) (Figures 1 and 2) in which the retention time in the chromatograms obtained from the quercetin standard sample and various concentrations of MO extract was compared (Figures 1 and 2). Then, using the standard curve and the resultant equation, the amount of quercetin was calculated at different concentrations of the extract (Table 1), and the amount of quercetin in one gram of plant leaf was 0.064 (mg/g).
To investigate the cytotoxicity effect of methanolic extract of Moringa olifera leaf on the HepG-2 cell line, cells were treated with various concentrations including 0, 30, 40, 50, 60, and 70 μg/mL methanolic extract and quercetin (50 μg/mL) and doxorubicin (1 μg/mL). Cell viability was assessed after 48h by MTT assay. The results showed that in all concentrations of methanolic extract the viability of liver cancer cells was significantly decreased (P ≤ 0.05) (Figures 3 and 4). IC50 for the methanolic extract of leaf of this plant was 12.89 μg/mL. Also, according to the results, the cytotoxicity effect of the highest concentration of extract (70 μg/ml) was similar to the doxorubicin and pure quercetin. Cell viability in the presence of doxorubicin was 11.5 ± 4.9%, in the presence of pure quercetin 15.1 ± 4.6% and in the presence of the extract at a concentration of 70 µg/mL, 11.97 ± 8%.
5. Discussion
Moringa olifera (MO) is a rich source of various nutrients and secondary metabolites such as phenolic compounds and flavonoids, glucosinolates, sterols, terpenoids, and thiocarbamates, which has several healing properties (15, 16). Keeping in view the anticancer properties of MO, in this study, we investigated the anticancer properties of methanolic extract of MO leaves against HepG2 (cancer liver cell) (4, 13). Various studies have reported the existence of phenolic compounds and about 12 types of flavonoids, including quercetin, kaempferol, isoramentine, and apigenin, in different parts of MO. But the amount of the flavonoid compounds in Moringa olifera is not similar in all cases, mainly due to climatic and geographical conditions differences where the plant grows as well as the differences in the methods of extraction, genetic diversity of plants, age difference leaves, and stage of maturity (17). In this study, the total flavonoids and quercetin in the leaves of MO were, respectively, 3.69 (mg/g) and 0.064 (mg/g (of dry weight, whereas the Coppin study reported that flavonoid components in sub-Saharan Africa MO methanolic extract were 0.11 to 1.26 g/100 g.
In the present study, MTT test results showed that the methanolic extract of the leaves inhibit the growth and proliferation of liver cancer cells. Also, the MO extract had a concentration-dependent cytotoxic effect on the Human hepatoma HepG2 cell line. As the concentration of the extract increased, the growth of the cancer cells was further inhibited. Following 48 h, IC50 values for HepG2 cells were found to be 12.89 µg/mL. Besides, the results indicated that the toxicity and anti-proliferative effect of the extract at some concentrations were similar to the anticancer drug doxorubicin and pure quercetin. Since the extract can contain different compounds, the cumulative effect of these substances can be greater than that of a single drug or compound. It should be noted that the concentration of the extract or drug may also affect the cytotoxic and anti-proliferative effects. In fact, extracts with lower or higher concentrations of MO extract may have different and unpredictable effects. The effect of methanol solvent alone was also examined using the in vitro MTT and the results showed that the cytotoxic effect of methanolic extracts was due to the extract compounds, not due to the solvent itself (data not are shown). The compounds in MO leaf extract can inhibit the enzymes and proteins that are abnormally activated in cancer cells and cause unnecessary proliferation. Identifying and proving the existence of flavonoids and quercetin, as one of the components in the leaf extract of this plant, may partly explain the anticancer effects of this plant.
On the other hand, cyclooxygenase enzymes (Cox), especially Cox-2, play an important role in processes such as inflammation and carcinogenesis. The inhibitory effect of Cox-2 is a useful method to prevent and treat cancer (18). Studies have shown that flavonoid antioxidants, such as quercetin, play an important role in the inhibition of these enzymes and maybe a good alternative to anti-inflammatory drugs such as ibuprofen (19). Quercetin, an important pharmacological member of the flavonoid family, is one of the most important antioxidants. Research have shown numerous medicinal effects of quercetin, such as protection against various diseases, including osteoporosis, some malignant tumors, and cardiovascular and pulmonary disorders (20). From another point of view, various research have pointed to the mechanisms mediating the anticancer effects of quercetin, such as antioxidant effects, binding to the Aryl hydrocarbon receptor (Ahr), reacting with intracellular messenger systems, and altering the signaling pathway (21). Activation of these receptors results in the suppression of several oncogenic signaling pathways. Quercetin may activate the p53 protein and induces mitochondrial apoptosis in tumor cells, finally leading to apoptotic cell death (22).
Yoshida et al. showed that polyhydroxylated flavonoids and quercetin inhibited in vitro cell growth of human malignant gastric cancer cell lines and reduced the rate of DNA synthesis specifically in the cancer cell line by 14%, compared to the normal human cell line. These compounds also caused cell growth arrest by stopping the transition from the G1 stage to the S phase of the cell cycle (1).
Stage G1 has a manager role in animal cell proliferation, and the G1 cycle cell step is different between normal and malignant cells, so any compound that can prevent the passage of G1 to S can be identified as a potential factor in controlling the growth of cancer tumors (23, 24). Malignant cells appear to be unable to bypass quercetin-dependent cell cycle arrest. Quercetin is an inhibitor of the epidermal growth factor receptor (EGFR) and Focal Adhesion Kinase (FAK) (25). These two proteins are essential for the growth and proliferation of cancer cells. Furthermore, quercetin induces the expression of genes involved in detoxification and can reduce the expression of other transcription factors (26). Quercetin also suppresses the activity of topoisomerase I and II, which play a key role in many malignancies (27). In addition to the quercetin, the extract contains other flavonoid compounds, such as kaempferol, that are proved to have an anticancer role by studying different cancer cell lines (15, 26). Therefore, it may be possible to attribute the anticancer effects of the extract of Moringa olifera to kaempferol.
Overall, the results of this study showed that the methanolic extract of Moringa olifera, with numerous flavonoid compounds, decreased the survival of liver cancer cells. However, the effect of this extract on healthy human cells was not investigated in this study, so it is necessary to be investigated. Additionally, it seems, identifies, and quantifies the effective compounds in Moringa olifera grown in different regions of Iran.