1. Background
Breast cancer, a type of malignancy that originated from breast tissue, is the leading type of cancer in women and the second leading cause of cancer-related deaths in women worldwide (1, 2). Breast cancer patients with overexpression of human epidermal growth factor receptor type 2 (HER2) commonly have low rates of disease-free survival, resistance to chemotherapy and hormone treatment, and high metastasis and mortality (3). Approximately in 20–30% of breast cancer patients, the HER2 is overexpressed (4, 5). Most chemical therapeutics is based on inducing apoptotic death to reduce the proliferation of cancerous cells (6). One of the common features of cancerous cells is to engage different mechanisms to escape apoptosis. Bcl-2 family proteins are highly involved in the intrinsic apoptosis pathway that is often triggered in response to chemotherapeutic agents (7, 8). The Bcl-2 family proteins like Bax and Bcl-2 have pro-apoptotic and anti-apoptotic activities, respectively.
Lapatinib is an orally anti-proliferative drug that is used for the treatment of breast cancer and other solid tumors. It is a dual tyrosine kinase inhibitor of HER2 kinases and epidermal growth factor receptor (EGFR) (9, 10). Lapatinib inhibits activation of HER2 and can suppress their major downstream signaling proteins such as Akt and extracellular signal-regulated kinases (ERKs) 1/2 (11, 12). Some studies reported that lapatinib can inhibit cell proliferation and promote apoptotic death by down-regulating Bcl2 and up-regulation of Bax, cleaved caspase-3, and cleaved caspase-9 expression (13). Unfortunately, primary or acquired resistance to lapatinib and multiple off-target side effects still occurs when treating breast cancer (14).
Metformin is the first-line medication for type 2 diabetes (15). Some studies have argued that metformin may prevent the proliferation of cancer cells (16). For breast cancer patients, metformin can decrease breast cancer cell viability via increasing the level of reactive oxygen species (ROS) (17, 18). Since the effects of lapatinib and metformin on cell viability and apoptotic death have been investigated in some studies (19, 20).
2. Objectives
The current study aimed to investigate the cytotoxic effects of metformin in combination with lapatinib on the cell viability and expression of Bax in the SKBR3 human breast cancer cell line as a HER2 overexpress cell line.
3. Methods
3.1. Cell Culture and Cell Viability Determination
The SK-BR3 cells were obtained from the Pasteur Institute of Iran (Tehran, Iran). The cells were cultured in DMEM containing 10% fetal bovine serum (FBS) and 0.5% pen-strep at 37°C. Approximately 5 × 103 cells were seeded in 96-well plates and allowed to receive 70% confluence. To assess the cytotoxic effect of metformin and lapatinib, the cells were treated with different concentrations of metformin (5-80 mM) and lapatinib (50-600 nM) alone and in combination for 48 hours, following the principles followed by previous studies (21, 22). The cells of the control group were left untreated. Therefore, four groups, including untreated, treated with lapatinib, treated with metformin, and treated with the lapatinib-metformin combination, were assessed in the current study. After 48 hours, 20 µl of MTT (3-(4, 5-dimethilthiazol-2yl)-2,5-diphenyl tetrazolium bromide) Sigma- Aldrich (St. Louis, MO, USA) solution (1 mg/ml) was added and incubated at 37 °C for 4 h. After solubilizing the blue formazan crystal in 100 µl DMSO at 37°C for 30 min, the absorbance was measured at 570 nm using BioTek ELX800 microplate reader (Winooski, United States). The percentage of viable cells was calculated as:
(O.D. of drug-treated sample/control O.D.) ×100.
This project was approved by the Ethics Committee at Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (IR.AJUMS.REC.1397.916).
3.2. Western Blotting
The gathered cells were lysed with the cold RIPA buffer containing 1% protease inhibitor cocktail (Thermo, Rockford, IL) for 20 min. Then, all cells were centrifuged at 12000 rpm for 15 min at 4°C. The total protein content of the lysates was determined using a BCA protein quantification kit. The protein samples (60 µg) were electrophoresed on a 12% polyacrylamide gel (SDS-PAGE) and then transferred to the PVDF membrane. Afterward, the membranes were blocked with 5% skim milk for 1 h, probed with primary anti-Bax antibody (Abcam Inc, Cambridge, UK, ab32503, 1:1000 dilution) and anti-Beta-actin as internal control (Cell Signaling Technology, Danvers, MA, USA, 4970 L, 1:2000 dilution) antibodies overnight at 4°C. Then, the membranes were incubated with the secondary goat anti-rabbit antibody (Cell Signaling Technology, Danvers, MA, USA, 7074s, 1:10000 dilution) for 1 h at room temperature. Finally, the membranes were incubated with ECL detection reagent to visualize the protein band by Bio-Rad ChemiDocTM (Hercules, CA, USA).
3.3. Statistical Analysis
Statistical analyses were performed using SPSS. Data are expressed as mean ± standard error of the mean (S.E.M) for three independent experiments. The comparisons of mean variables between groups were performed using the One-way ANOVA followed by the Tukey post hoc test.
4. Results
4.1. Cytotoxic Effect of Lapatinib and Metformin on the SKBR-3 Cells
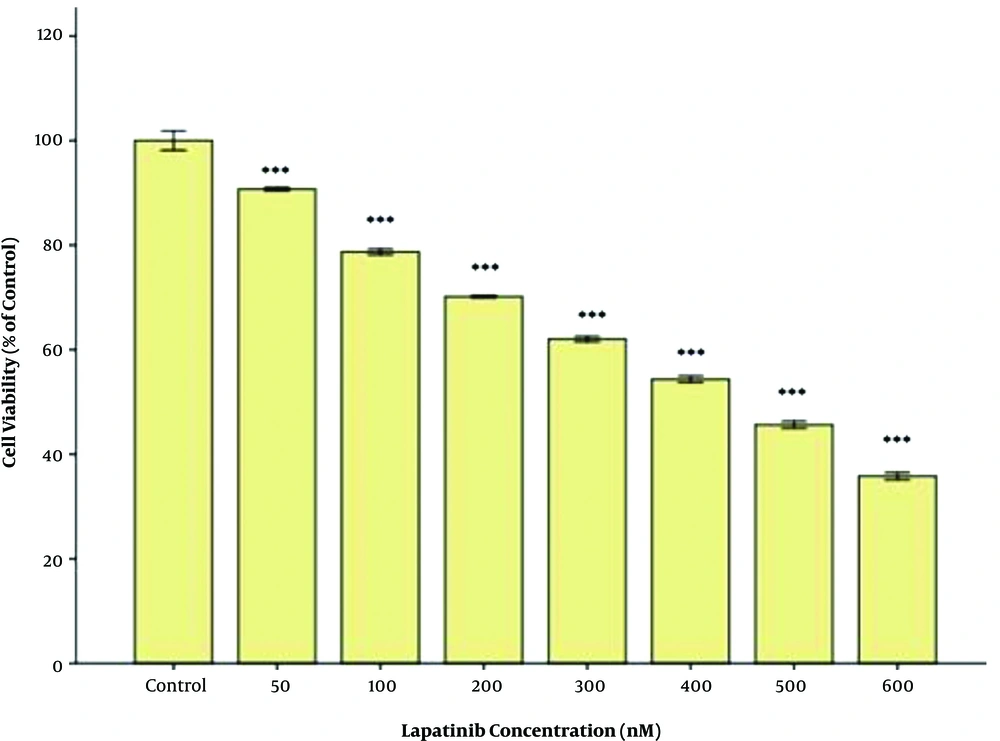
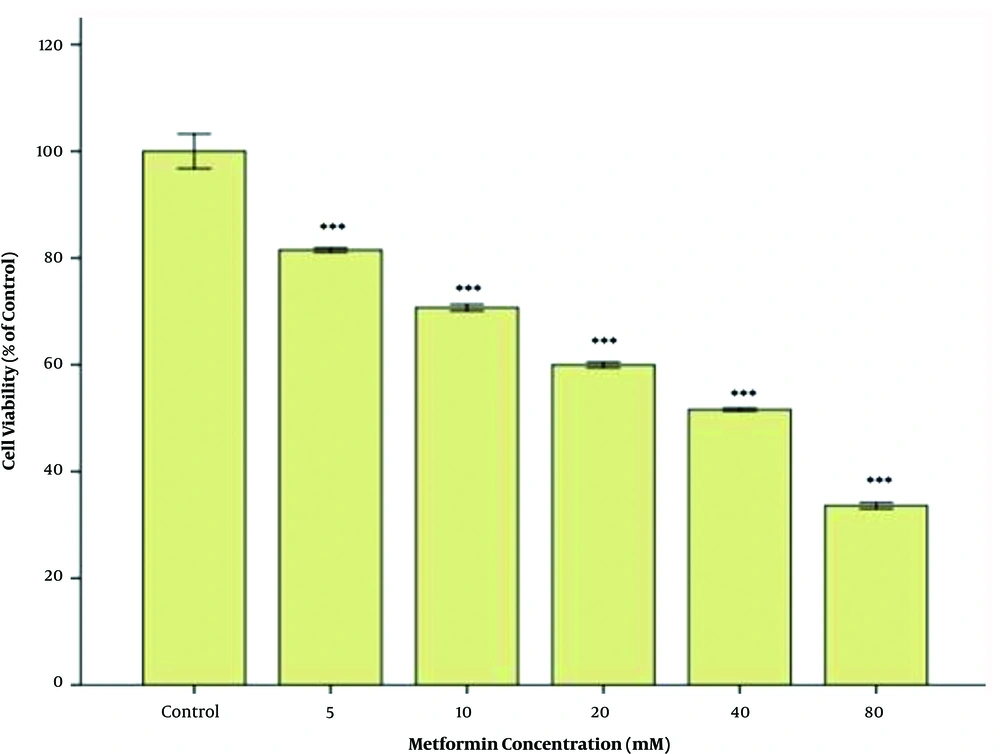
The inhibitory effect of lapatinib on the cell viability was assessed, and the least cytotoxicity was observed after 48h of treatment at 50 nM. The dose-dependent inhibitory effect of lapatinib on cell viability was evaluated based on its IC50. The results are briefly provided in Figure 1. After 48 h of treatment with lapatinib, the value of IC50 on the cell viability was estimated at approximately 250 nM (Figure 1). The inhibitory effect of metformin on the cell viability was assessed, and its least cytotoxicity was observed after 48 h of treatment at 5 mM. The dose-dependent inhibitory effect of metformin on the cell viability was evaluated based on its IC50 and the results are briefly provided in Figure 2. After 48 h of treatment with metformin, the value of IC50 on the cell viability was estimated at approximately 40 mM (Figure 2).
4.2. Cytotoxic Effects of the Metformin-Lapatinib Combination on the SKBR-3 Cells
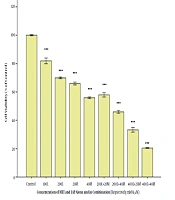
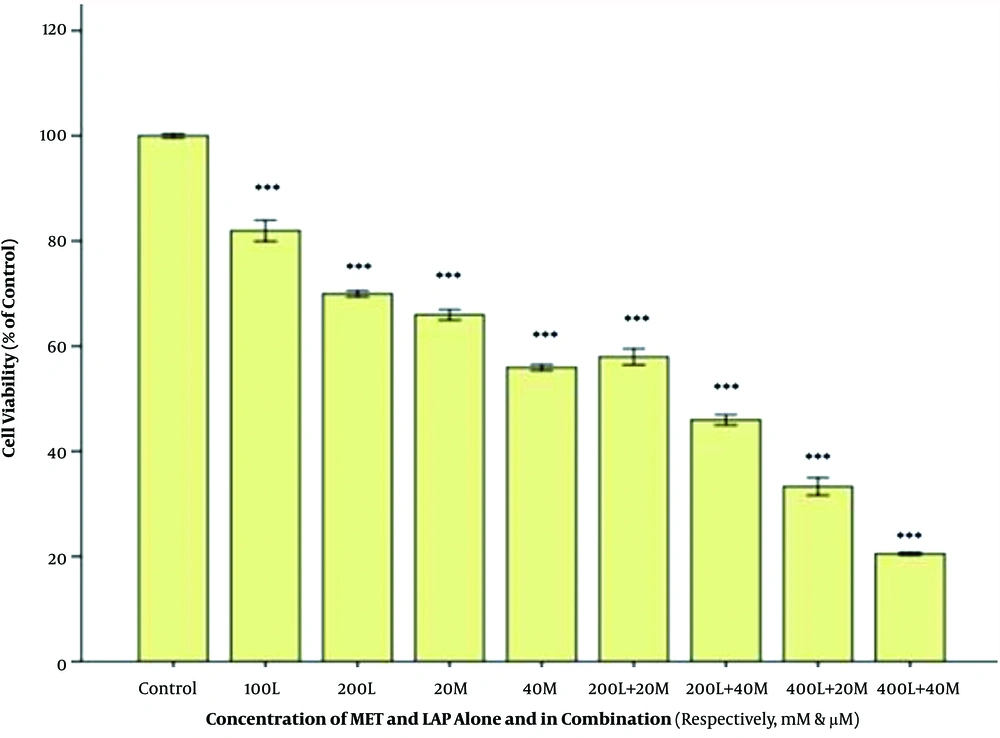
To determine whether metformin can enhance the efficacy of the cytotoxicity effect of lapatinib against breast cancer cell lines, we assessed the inhibitory effect of the metformin-lapatinib combination on the cell viability of SKBR3 cells. As presented in Figure 3, the metformin-lapatinib combination showed a higher inhibitory effect on the cell viability of SKBR3 cells compared to lapatinib used individually. A co-treatment of 20 and 40 mM of metformin with 200 nM of lapatinib at 48h caused 40% and 50% inhibition, respectively, as compared to sole administration of lapatinib, which showed approximately 30% inhibition. The highest inhibition (approximately 80%) was observed in co-treatment of 40 nM of metformin and 400 nM of lapatinib compared to when lapatinib was used alone, which showed approximately 58% inhibition (Figure 3).
The cytotoxic effect of the Metformin-lapatinib combination on the SK-BR3 cells. After treatment with various concentrations of metformin-lapatinib combination for 48 h, the cells were subjected to MTT assay, and the controls remained untreated. The results are provided as mean ± S.E.M. ***P < 0.001 vs. control. L= lapatinib, M = metformin
4.3. Effect of the Metformin-Lapatinib Combination on the Bax Expression
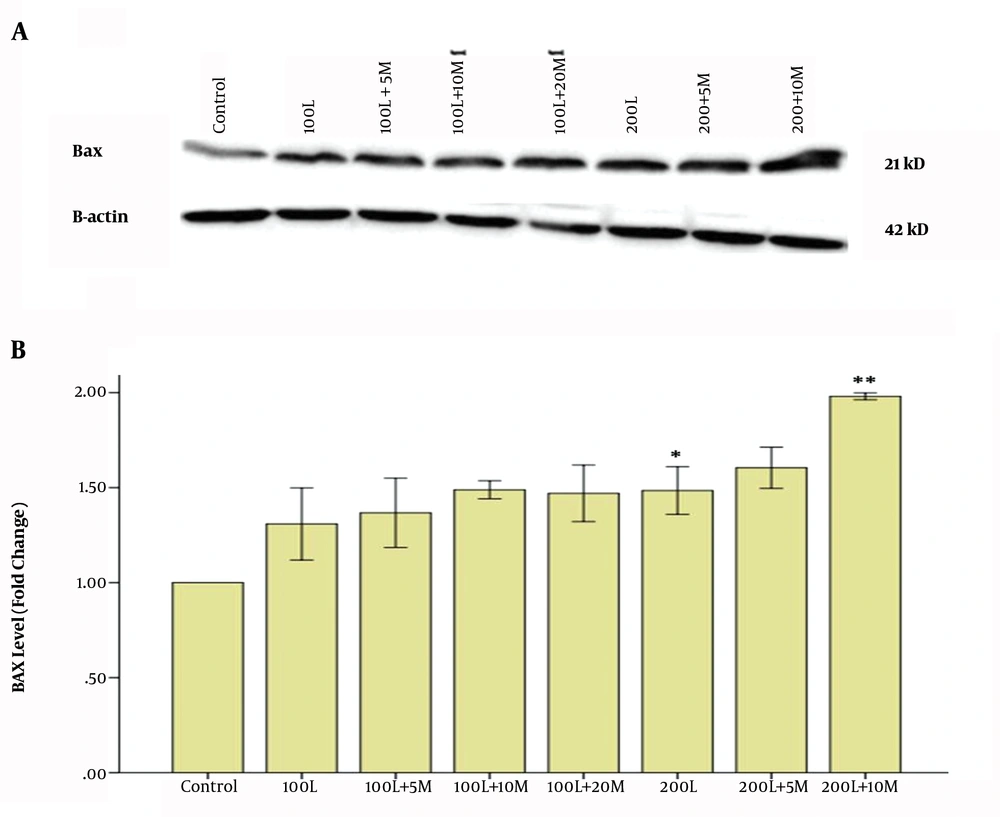
Finally, we assessed the effect of the metformin-lapatinib combination on the expression of pro-apoptotic protein Bax in SKBR-3 cells. The results indicated that when the cells were treated with 100 nM of lapatinib alone, the expression of Bax was significantly increased in comparison to the control cells (Figures 4A and B). Also, the results showed that when the cells were co-treated with 10 mM of metformin plus 200 nM of lapatinib the expression of Bax was significantly increased in comparison to the cells that were treated with 200 nM of lapatinib alone (Figure 4A).
Effect of Metformin-lapatinib combination on the expression of Bax (A). The cells were treated with 100 and 200 nM of lapatinib alone and various concentrations of the metformin-lapatinib combination for 48h. In the current experiment, the concentrations of metformin were 5, 10, and 20 mM, respectively. Image j software was used to determine the levels of Bax after Western blotting (B). L = Lapatinib; M = Metformin. The results are provided as the mean ± S.E.M. *P < 0.05 vs. control; ** P < 0.01 vs. 200L.
5. Discussion
Nearly 25 to 30% of breast cancer patients experience overexpression of the human epidermal growth factor receptor gene (HER2), and its encoded protein presents in the malignant cells. Those with overexpressed HER2 experience severe forms of breast cancer with reduced disease-free survival (3, 23). Various treatments are currently available for breast cancer patients, among which chemotherapy has the most severe side effects on patients' quality of life (24). Hence, developing a novel therapeutic option with lower side effects is of crucial importance. Lapatinib is widely prescribing for breast cancer patients with overexpressed HER2. However, caution should be taken when prescribing lapatinib for breast cancer patients who receive chemotherapy, particularly regarding safety measures in the qualification for treatment and identification of potential complications are necessary. Heart failure and cardiotoxicity are the most common side effects of lapatinib treatment, which are associated with decreased left ventricular ejection fraction (LVEF) (25). Some studies mentioned metformin, an anti-diabetic drug that helps to prevent cancer progression, as a safe drug (16). Moreover, we reported that co-treatment of SK-BR3 cells with combined lapatinib-metformin showed a more potent cytotoxic effect than sole treatment with each drug. After treatment with 500 nM of lapatinib for 48 hours, the cell viability of SK-BR3 was reduced by approximately 50%. While the viability was reduced approximately to 50% after administering 200 nM of lapatinib in combination with 40 mM of metformin after 48 hours. As reported by Vazquez-Martin et al., metformin can both stimulate AMP-activated kinase (AMPK) and reverse lapatinib resistance in MCF-7/HER2 LapR cells. A 2.5 mM concentration of metformin increased the sensitivity of MCF-7/HER2 LapR cells to lapatinib by twofold, while a concentration of 10 mM decreased lapatinib IC50 (26).
Previous studies reported significant reductions in breast cancer progression after administering metformin in animal models with overexpression of HER2 (27, 28). It's well-documented that metformin can promote cell cycle arrest in the G1 phase and induces apoptosis in a wide variety of breast cancer cell lines (29). According to Hangjun Gong et al., metformin selectively induced apoptosis in human gastric cancer cells but did not affect the non-cancerous cells (30). The results of the present study showed that in the SKBR-3 breast cancer cell line, metformin at 20 mM combined with lapatinib could significantly increase the levels of pro-apoptotic Bax compared to lapatinib alone. Therefore, metformin synergistically induces a lapatinib-mediated cytotoxic effect on cell death and apoptosis. Bax, a pro-apoptotic protein of the BCL2 family, can interact with and enhances the opening of the mitochondrial voltage-dependent anion channel, which led to changes in the mitochondrial membrane permeability and release of cytochrome C. The expression of Bax is commonly regulated by the tumor suppressor P53 and may be involved in the P53 mediated cell apoptosis (31). Li P et al. reported that treatment of MCF-7 cells with metformin increased the level of p53 protein and its targets, including Bax and p21. Also, metformin plays an important role in regulating P53 through AMPK-mTOR signaling. AMP-activated protein kinase (AMPK) activation, a key cellular energy sensor kinase, leads to decreased mTOR signaling, progression, and proliferation (32). Alex J Eustace et al. showed that the changes in the members of the Bcl-2 family, especially Bax, may have a key role in resistance to lapatinib, which occurs in positive HER2 tumors (33).
5.1. Conclusion
This study demonstrated that the metformin-lapatinib combination could synergistically inhibit the cell viability of SKBR3 cells. These in-vitro cytotoxic effects of metformin and lapatinib alone and in combination, may be mediated by increasing the level of pro-apoptotic BAX protein expression. These results confirm the possible advantage of metformin-lapatinib combination for intervention in breast cancer patients with HER2 overexpression.