1. Background
The endocannabinoid system (ECS) is expressed throughout the human body and plays important roles in a variety of physiological functions. Its ability to modulate inflammation and the immune response is remarkable, thereby controlling ECS may affect the outcome of several pathologies (1), in particular, gastrointestinal diseases (2). This system consists of three parts, including endogenous ligands, receptors, and enzymes responsible for synthesis and degradation of cannabinoids and endocannabinoids (3). Cannabinoid receptors 1 (CB1Rs) that located throughout the central, peripheral, and enteric nervous system (4) can be found all over the gastrointestinal tract (GI) (5). Cannabinoid receptors 2 (CB2Rs) are mainly expressed in immune cells, especially macrophage lineage (6). The endocannabinoid system is a powerful regulatory system in the GI involved in some crucial activities such as motility, secretion, inflammation and carcinogenesis (7).
Gastric cancer is one of the most common cancers and the third cause of death worldwide (8). Chronic inflammation has been identified as one of the main causes of gastric cancer (9). Stomach infections, such as Helicobacter pylori and chronic gastritis, present strong etiological links between inflammation and gastric tumorigenesis (9). CB1R is partly responsible for the regulation of inflammatory responses. The stomach is described as the main source of CB1R (10). Some researchers have reported misregulated expression of CB1R in various types of cancers such as breast (11), and liver cancers (12). Inflammation is crucial to cancer development and promotes all stages of tumorigenesis. Pro-inflammatory cytokines such as interleukin (IL)-6 and IL-1β are implicated in inflammation-induced tumorigenesis, in particular, in the intestine (13). However, there is no data available about the involvement of inflammation in CB1R expression pattern and the promotion of cell growth in gastric cancer.
2. Objectives
The purposes of this study were to evaluate the CB1R expression in human gastric cancer, mRNA and protein expression of CB1R under LPS-mediated inflammation condition in human gastric cancer cells (AGS), and the effects of inflammation on cell proliferation in LPS-stimulated AGS cells.
3. Methods
3.1. Tissue Specimens
According to the previous studies to determine the expression level of cannabinoid receptors in various cancer tissues (14, 15), a total of 20 pairs of human gastric cancer and normal adjacent tissue samples from patients who previously underwent gastric cancer surgery were obtained from Iran National Tumor Bank (INTB), Cancer Institute, Tehran University of Medical Sciences, according to procedures approved by the Ethics Committee at the INTB (approval no: INTB, IR138).
The inclusion criteria were newly diagnosed gastric cancer with defined clinicopathological characteristics, patient information (geographical location, age, sex, sampling date), and clinical metastasis. The patients with other systemic diseases such as infections, diabetes, cardiovascular and neuroscience, and those under any treatment such as anti-inflammatory and anticancer therapy were excluded from the study.
The clinical characteristics of the samples used in this study are listed in Table 1. Written informed consent was obtained from all enrolled patients or close relatives.
| Parameter | Subjects, No. (%) |
|---|---|
| Tumor stage | |
| II | 3 (15) |
| III | 12 (60) |
| IV | 5 (25) |
| Tumor grade | |
| I | 6 (30) |
| II | 3 (15) |
| III | 11 (55) |
| Tumor size (cm) | |
| ≤ 5 | 8 (40) |
| > 5 | 12 (60) |
| Histology | |
| Invasive | 4 (20) |
| Adenocarcinoma | 13 (65) |
| Carcinoma | 3 (15) |
Characteristics of Gastric Cancer Samples Examined in This Study
3.2. Cell Line and Culture
Human gastric cancer cell line AGS was purchased from Cell Bank of Pasteur Institute (Tehran, Iran), and was cultured with RPMI-1640 containing 10% FBS and 1% penicillin-streptomycin (Gibco, Carlsbad, CA, USA) in a CO2 incubator at 37°C and 5% CO2.
3.3. Lipopolysaccharide–Induced Inflammation in AGS Cells
Inflammation was induced using LPS (O111.B4, Sigma-Aldrich, MO, USA) as described previously (16). In brief, AGS cells were seeded in a 6-well plate at a density of 7 × 105 cells/well and were cultured overnight, then they were treated with LPS at the dose of 1 and 10 µg/ml for 6 h to select the proper dose to evoke pro-inflammatory responses. The selection of doses and incubation time was based on reported data (17-19). Induction of inflammation was confirmed by analysis of the mRNA levels of IL-1β, TNF-α, and IL-6 as important inflammatory mediators in AGS cells by specific primers listed in Table 2 using quantitative real-time polymerase chain reaction (qRT-PCR).
| Gene Name | Forward Sequence (5’-3’) | Reverse Sequence (5’-3’) | Length, bp |
|---|---|---|---|
| β-actin | GAGCATCCCCCAAAGTTCACA | GGGACTTCCTGTAACAACGCA | 103 |
| CB1R | TGAACTCCACCGTGAACC | CTGTGAACACTGGCTGCA | 169 |
| IL-1β | ACGAATCTCCGACCACC | CTCGTTATCCCATGTGTCGAAG | 184 |
| IL-6 | GTGTGAAAGCAGCAAAGAGGC | TACCTCAAACTCCAAAAGACCAGTG | 142 |
| TNF-α | AGCCCATGTTGTAGCAAACC | ATGAGGTACAGGCCCTCTGA | 135 |
Sequences of Primers Used in qRT-PCR
3.4. RNA extraction and real-time PCR
The total RNA from tumor, normal tissues, and AGS cells were processed using TRIzol solution (TaKaRa Bio, Tokyo, Japan), according to the manufacturer’s protocol. First-strand cDNA was synthesized from 2 µg RNA using Revert Aid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific Inc, Massachusetts, USA) with oligo (dT) and random hexamer primers according to manufacturer’s protocol. The expression of CB1R, IL-1β, tumor necrosis factor α (TNF-α), and IL-6 at transcriptional level were assayed using primers shown in Table 2. Moreover, β-actin was utilized as an internal control. Real-time PCR was performed using the Roche Light-Cycler detection system (Basel, Switzerland) by the qPCRTM Green Master Kit for SYBR Green I® (Yektatajhiz, Tehran, Iran). The relative expression level of the target genes was compared to β-actin, reactions were performed in triplicate. Two separate reactions without cDNA or with RNA were performed in parallel as controls. Relative quantification was performed according to the comparative 2-ΔΔCt method, using Lightcycler 96® software. Validation of assay to check whether the primers of target genes and housekeeping gene had similar amplification efficiencies was performed as described previously, and all qPCR analysis was performed according to The Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guideline (20).
3.5. Western Blot Analysis
Total proteins were extracted from AGS cells using RIPA lysis buffer (Tris-HCl 50 mM, NaCl 150 mM, Triton X-100 0.1%, NaF 1mM) supplied with protease inhibitor cocktails (Sigma-Aldrich, MO, USA), and the protein concentration was measured by Bradford assay. An equal amount of proteins (10 µg per well) were loaded onto 10% SDS-PAGE gel, followed by electrophoresis, and transferred onto PVDF membranes (EMD, Millipore). The membranes were blocked in 5% skim milk for 1 h at room temperature. After washing with PBS-T buffer, co-incubated with anti-CB1 (1:500; sc-518035, Santa Cruz, USA), and anti-β-actin (1:300; sc-47778, Santa Cruz, USA) overnight at 4°C. After washing, membranes were co-incubated with secondary antibody m-IgGκ (1:1000; sc-516102, Santa Cruz, USA) on a shaker at room temperature for 2 h. After washing, the membranes were developed using an ECL kit (Thermo Scientific; Waltham, USA). Protein expressions were normalized to β-actin.
3.6. 5-Bromo-2-Deoxyuridine (BrdU) Labeling Cell Proliferation Assay
To determine the effect of LPS on the proliferation of AGS cells, 5-bromo-2’-deoxyuridine (BrdU) labeling and detection kit (Roche, Basel, Switzerland) was used according to the manufacturer’s protocol. In brief, after treatment with LPS for 6 h, AGS cells were cultured for 24 h. The following day, the culture medium was aspirated, and the BrdU reagent was diluted at a ratio of 1:1000 (final concentration 10 µM) and added to the cells (100 µL/well), then the cells were incubated at 37°C in a 5% CO2 incubator for 60 min. After washing with washing buffer, the cells were fixed using 200 µL/well ethanol fixative solution for at least 30 min at -20°C. The cells were washed, and an anti-BrdU-POD monoclonal antibody (dilution, 1:10) was added (100 µL/well) to the cells and incubated for 90 min at 37°C. Following washing, anti-mouse-Ig-fluorescein (dilution 1:10) was added (100 µL/well) to the cells and incubated for 30 min at 37°C. The cells were washed and detected in the range of 515 - 565 nm using a fluorescence microscope. The number of BrdU-positive staining cells was obtained by counting labeled cells per 100 cells under a fluorescence microscope (Zeiss, Oberkochen, Germany). Each experiment was repeated three times, and the counting was taken in triplicate for each experiment.
3.7. Statistical Analysis
All experiments reported in the present study were performed at least in triplicate. The data were reported as mean ± standard error of mean (SEM). The statistical significance of the difference between groups was compared using the Student t-test. Differences with P < 0.05 were considered statistically significant.
4. Results
4.1. CB1R Was Overexpressed in Human Gastric Cancer
Data showed that the mRNA expression level of CB1R was upregulated in gastric cancer samples compared with that of matched adjacent non-tumor tissues from gastric cancer patients (Figure 1). CB1R showed an increase of 4.63-fold in gastric cancer samples (P < 0.05).
4.2. Inflammation Was Induced by LPS in AGS Cells
As shown in Figure 2, mRNA levels of IL-1β, IL-6, and TNF-α genes were significantly increased following LPS treatment (10 µg/mL, 6 h) (P < 0.05). This finding indicated that LPS at the dose of 10 µg/mL was a more potent immunostimulant than 1 µg/mL. So, this concentration of LPS was selected for subsequent experiments.
Effect of LPS stimulation on the expression of IL-1β, IL-6, and TNF-α in AGS cells. The cells were stimulated with 1 and 10 µg/mL LPS for 6 h and the expression levels of genes were evaluated using qRT-PCR analysis. Data were normalized to β-actin and were expressed as means ± SEM. All experiments were performed in triplicate. **, P < 0.01; ***, P < 0.001.
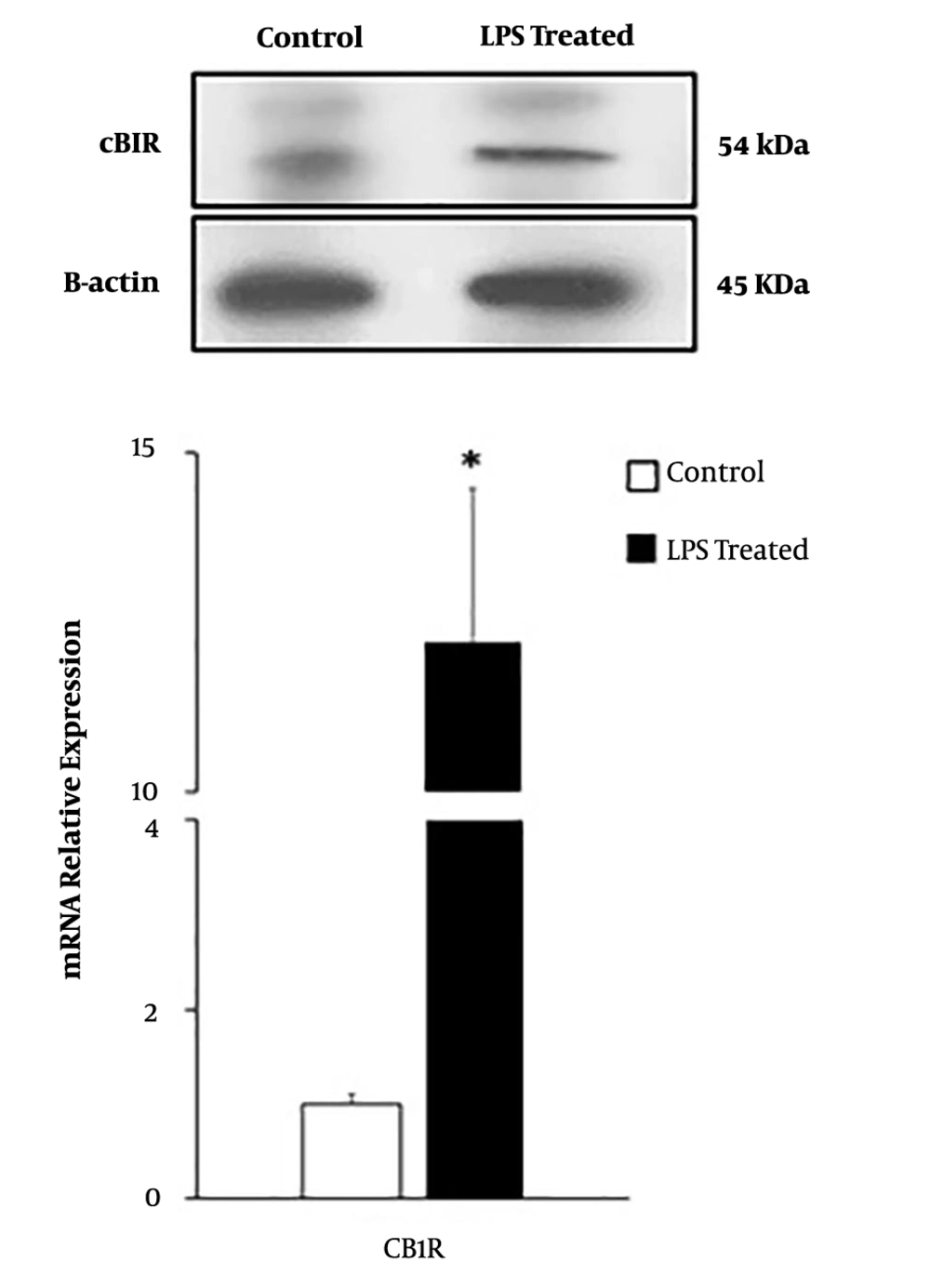
4.3. The effect of LPS Treatment on the Expression of CB1R in AGS Cells
In this study, qRT-PCR and western blot data demonstrated that AGS cells expressed CB1R. LPS induction significantly enhanced CB1R expression at mRNA and protein levels in AGS cells (P < 0.05) (Figure 3).
4.4. LPS Boosted Cell Proliferation in AGS Cells
BrdU assay was used to assess the cell proliferation rate of AGS cells after treatment with LPS. As shown in Figure 4, the proliferation of LPS-stimulated AGS cells showed a significant increase of 13% after 24 h (P < 0.05).
Effect of LPS treatment on cell proliferation in AGS cells. LPS treatment promoted cell proliferation in AGS cells compared with the control group. A, Representation of cell proliferation under a fluoresence microscope; B, the number of BrdU-positive staining cells was obtained by counting labeled cells per 100 cells under a fluorescence microscope in the LPS-treated group compared with the control group. Each experiment was performed in triplicate. *, P < 0.05, in comparison to the control group.
5. Discussion
In the present study, the expression of CB1R in gastric cancer tissues and non-tumor adjacent tissues, as well as in gastric cancer cell line AGS, were evaluated. The results showed that CB1R was overexpressed in human gastric cancer samples compared to adjacent non-tumor samples and also demonstrated the expression of CB1R in AGS cells. Moreover, the findings indicated the effect of LPS on upregulation of CB1R and cell proliferation in AGS cells.
Recent studies revealed the aberrant expression of cannabinoid receptors, in particular CB1R, in several tumors (21). It has been reported that CB1R and CB2R were upregulated in biopsies taken from patients with prostate cancer compared to healthy individuals (22). Consistent with our results and according to the study was conducted by Xian et al. (23), CB1R was overexpressed in gastric cancer tissue samples. However, the molecular mechanisms underlying this alteration have not been fully elucidated. It is confirmed pro-inflammatory mediators stimulate CB1R and CB2R expression in the cells treated with inflammatory molecules (24), and mediate an increase in cannabinoid receptors from an initial steady-state to a second higher state through common mechanisms (25). We demonstrated the expression of CB1R by qRT-PCR and western blot in AGS cells. In this study, our findings showed LPS treatment could upregulate CB1R expression in AGS cells, and we concluded the overexpression of CB1R in gastric cancer tissues may be a result of inflammation. The involvement of CB1R in the inflammatory responses was confirmed for the first time in dorsal root ganglia (DRG), where CB1R was upregulated after the treatment with Freund’s adjuvant (26).
Our findings revealed that cell proliferation was increased following LPS stimulation in AGS cells. Earlier researches demonstrated the effects of IL-1β (27), and other inflammatory cytokines (28) in gastric cancer progression and showed that upregulation of CBRs led to the activation of downstream pathways involved in cell progression and survival and cancer cell invasion through some pathways such as PI3K/PKB phosphorylation (29, 30), ERK cascade (31), and CXCR4/CXCL12 (32). Taken together, previous results and findings suggest that inflammatory cytokines may exert their function through upregulation of CB1R. In addition, overexpression of CB1R may play a role in the development and progression of gastric cancer.
In conclusion, we found that human gastric cancer tissues and AGS cells both overexpressed CB1R, and LPS could enhance CB1R expression at mRNA and protein level, and it promoted cell proliferation in AGS cells. Our results suggest that CB1R may be a promising candidate for diagnostic and therapeutic aims in gastric cancer.